2,2'-Bipyridine
Synonym(s):2,2′-Bipyridine;2,2′-Bipyridyl;2,2′-Dipyridine;2,2′-Dipyridyl;Reagents for Iron(II) and molybdenum, α,α′-Dipyridyl
- CAS NO.:366-18-7
- Empirical Formula: C10H8N2
- Molecular Weight: 156.18
- MDL number: MFCD00006212
- EINECS: 206-674-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
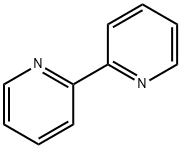
What is 2,2'-Bipyridine?
Chemical properties
White to light red crystalline powder. Soluble in ethanol, ether, benzene, chloroform and petroleum ether, 1 part of this product is about 200 parts of water.
The Uses of 2,2'-Bipyridine
2,2'-bipyridine acts as a bidentate chelating ligand which form complexes with transition metal ions and shows antifungal, antibacterial and antiviral activity. It is used for the colorimetric determination of iron as well as oxidation reduction indicators to confirm the presence of ferrous ion in soils. It forms complexes with ruthenium and platinum, exhibit intense luminescence, which may have practical applications. Copper(I) bipyridine complexes involved in the oxidation of alcohols under aerobic conditions.
The Uses of 2,2'-Bipyridine
A high-affinity chelator of iron. A reagent used for the determination of iron.
Definition
ChEBI: 2,2'-bipyridine is a bipyridine in which the two pyridine moieties are linked by a bond between positions C-2 and C-2'. It has a role as a ferroptosis inhibitor and a chelator.
Preparation
2,2'-bipyridine is synthesized by the reaction of pyridine with ferric chloride. Take 70g of anhydrous pyridine and mix with 13g of anhydrous ferric chloride, heat and react at 300℃ in a sealed tube for about 35h. After cooling, the reactant solidified into red-black crystals, the sealing tube was opened, and the red-black solution of the solid was washed out with a small amount of hot water. The oily impurities were removed by extraction with ether. After neutralization with sodium bicarbonate, excess pyridine was removed by heating with steam. The mixture was then made strongly basic, and the 2,2'-bipyridine was distilled off with steam.
Reactions
A large number of complexes of 2,2'-bipyridine have been described.It binds metals as a chelating ligand, forming a 5-membered chelate ring.
Synthesis Reference(s)
Journal of the American Chemical Society, 100, p. 5567, 1978 DOI: 10.1021/ja00485a053
Synthesis, p. 564, 1986 DOI: 10.1055/s-1986-31705
General Description
2,2′-Bipyridyl, is a symmetrical bipyridine commonly used as a neutral ligand for complexation with metal ions. The molecule is planar with trans-conformation and crystallizes in the monoclinic crystal system.
Biochem/physiol Actions
Metalloprotease inhibitor, high-affinity chelator of iron; may inhibit Fe2+ containing enzymes at 10?8?M.
Safety Profile
Poison by ingestion,subcutaneous, and intraperitoneal routes. Experimentalteratogenic data. Questionable carcinogen withexperimental tumorigenic data. Mutation data reported.When heated to decomposition it emits toxic fumes ofNOx.
Source
2,2'-Bipyridine is a natural product found in Dichilus strictus, Dichilus gracilis, and other organisms with data available.
Purification Methods
2,2'-Bipyridyl crystallises from hexane, or EtOH, or (after charcoal treatment of a CHCl3 solution) from pet ether. Also, it precipitates from a concentrated solution in EtOH by addition of H2O. Dry it in a vacuum over P2O5. It can be further purified by chromatography on Al2O3 or by sublimation. UV (EtOH): at 280nm (log 4.13). max [Airoldi et al. J Chem Soc, Dalton Trans 1913 1986, Beilstein 23 H 199, 23/8 V 16.]
Properties of 2,2'-Bipyridine
| Melting point: | 70-73 °C(lit.) |
| Boiling point: | 273 °C(lit.) |
| Density | 1.1668 (rough estimate) |
| vapor pressure | 0.584Pa at 25℃ |
| refractive index | 1.4820 (estimate) |
| Flash point: | 121 °C |
| storage temp. | room temp |
| solubility | 5.5g/l |
| form | Crystalline Powder |
| pka | pK1:-0.52(+2);pK2:4.352(+1) (20°C) |
| color | White to almost white |
| Odor | Characteristic odour |
| PH | 7.5 (5g/l, H2O, 25℃) |
| Water Solubility | 5.5 g/L 22 ºC |
| Merck | 14,3347 |
| BRN | 113089 |
| Stability: | Stable. Incompatible with strong oxidizing agents, most common metals. May be light sensitive. |
| CAS DataBase Reference | 366-18-7(CAS DataBase Reference) |
| NIST Chemistry Reference | 2,2'-Bipyridine(366-18-7) |
| EPA Substance Registry System | 2,2'-Bipyridine (366-18-7) |
Safety information for 2,2'-Bipyridine
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for 2,2'-Bipyridine
| InChIKey | ROFVEXUMMXZLPA-UHFFFAOYSA-N |
2,2'-Bipyridine manufacturer
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran


You may like
-
 366-18-7 2,2-Bipyridyl, 98% 98%View Details
366-18-7 2,2-Bipyridyl, 98% 98%View Details
366-18-7 -
 2,2'-Bipyridine CAS 366-18-7View Details
2,2'-Bipyridine CAS 366-18-7View Details
366-18-7 -
 2,2'-Bipyridine CAS 366-18-7View Details
2,2'-Bipyridine CAS 366-18-7View Details
366-18-7 -
 2, 2'-Bipyridyl CAS 366-18-7View Details
2, 2'-Bipyridyl CAS 366-18-7View Details
366-18-7 -
 2,2-Bipyridyl ACS CAS 366-18-7View Details
2,2-Bipyridyl ACS CAS 366-18-7View Details
366-18-7 -
 2,2'-Bipyridyl CAS 366-18-7View Details
2,2'-Bipyridyl CAS 366-18-7View Details
366-18-7 -
 2,2'-Bipyridyl, 98% CAS 366-18-7View Details
2,2'-Bipyridyl, 98% CAS 366-18-7View Details
366-18-7 -
 2,2'-Bipyridyl, GR 99%+ CAS 366-18-7View Details
2,2'-Bipyridyl, GR 99%+ CAS 366-18-7View Details
366-18-7
