2-Methoxynaphthalene
Synonym(s):2-Methoxynaphthalene;Methyl 2-naphthyl ether;Naproxen Impurity M
- CAS NO.:93-04-9
- Empirical Formula: C11H10O
- Molecular Weight: 158.2
- MDL number: MFCD00004061
- EINECS: 202-213-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31

What is 2-Methoxynaphthalene?
Description
β-Naphthyl methyl ether has an intensely sweet, floral odor suggestive of orange blossoms. It is free from naphthol by-odor. It has a sweet, strawberry taste. This may be prepared from potassium β-naphthol and methyl chloride at 300°C; by methylation of β-naphthol with dimethyl sulfate or by direct esterification with methyl alcohol.
Chemical properties
white powder
Chemical properties
Methyl 2-Naphthyl Ether forms white crystals (mp 73–74°C) with an intense orange blossom odor.
Chemical properties
β-Naphthyl methyl ether has an intensely sweet, floral odor suggestive of orange blossoms; free from naphthol by-odor. It has a sweet, strawberry taste
The Uses of 2-Methoxynaphthalene
2-Methoxynaphthalene is an Impurity of the non-steroidal anti-inflammatory Naproxen (N377525).
The Uses of 2-Methoxynaphthalene
2-methoxynaphthalene acylation is used as a model reaction to study the catalytic benefits of delamination. It was also used to study the alkali-metal-mediated manganation (AMMMn) reactions.
The Uses of 2-Methoxynaphthalene
2-methoxynaphthalene acylation was used as a model reaction to study the catalytic benefits of delamination. It was also used to study the alkali-metal-mediated manganation (AMMMn) reactions.
Definition
ChEBI: 2-Methoxynaphthalene is a member of naphthalenes.
Preparation
From postassium β-naphthol and methyl chloride at 300°C; by methylation of β-naphthlol with dimethyl sulfate or by direct esterification with methyl alcohol
Synthesis Reference(s)
Tetrahedron, 48, p. 6439, 1992 DOI: 10.1016/S0040-4020(01)88233-8
Tetrahedron Letters, 22, p. 3463, 1981 DOI: 10.1016/S0040-4039(01)81932-8
Flammability and Explosibility
Not classified
Synthesis
Preparation of 2-Methoxynaphthalene from 2-naphthol.
Principle: Phenols can be methylated to give methyl ethers. Methylation can be done either by using diazomethane or dimethyl sulphate in alkaline medium.
Reaction:
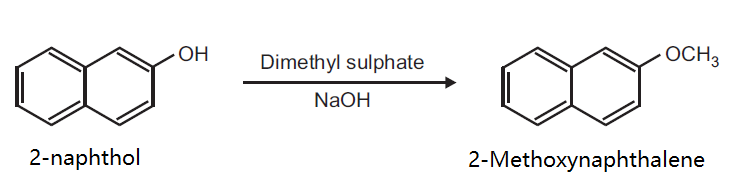
Procedure: Take 0.5 g 2-naphthol and 0.2 g NaOH in 5 ml distilled water in a beaker (25 ml). Heat on a wire gauze to obtain a clear solution. Cool the solution (10-15°C) and then add 0.35 ml dimethyl sulphate drop wise. After the addition is over, warm the mixture for one hour at 70-80°C and then cool. Filter the product and wash it with 10% sodium hydroxide solution and then with water. Dry the product, record the practical yield and re-crystallize it.
Re-crystallization: Dissolve the crude product in minimum amount of ethyl alcohol in a beaker by heating on a water bath. Filter the hot solution and cool the filtrate. Filter the white crystals of the product. Dry and record the melting point and TLC (using toluene as solvent).
Purification Methods
Fractionally distil the ether under vacuum. Crystallise it from absolute EtOH, aqueous EtOH, *C6H6, pet ether or n-heptane, and dry it under vacuum in an Abderhalden pistol or distil it in vacuo. The picrate has m 118o (from EtOH or CHCl3). [Kikuchi et al. J Phys Chem 91 574 1987, Beilstein 6 III 2969, 6 IV 4257.]
Properties of 2-Methoxynaphthalene
| Melting point: | 70-73 °C (lit.) |
| Boiling point: | 274 °C (lit.) |
| Density | 1.064 g/mL at 25 °C (lit.) |
| vapor pressure | 1.097Pa at 25℃ |
| refractive index | 1.5440 (estimate) |
| FEMA | 4704 | BETA-NAPHTHYL METHYL ETHER |
| Flash point: | 272-274°C |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | H2O: soluble (completely) |
| form | Crystalline Solid |
| pka | 0[at 20 ℃] |
| color | White to yellow-brown |
| Odor | at 1.00 % in dipropylene glycol. sweet naphthyl floral orange blossom acacia neroli |
| Water Solubility | INSOLUBLE |
| JECFA Number | 1257 |
| Merck | 14,5997 |
| BRN | 1859408 |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 93-04-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Naphthalene, 2-methoxy-(93-04-9) |
| EPA Substance Registry System | Naphthalene, 2-methoxy- (93-04-9) |
Safety information for 2-Methoxynaphthalene
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P391:Collect spillage. Hazardous to the aquatic environment P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for 2-Methoxynaphthalene
| InChIKey | LUZDYPLAQQGJEA-UHFFFAOYSA-N |
2-Methoxynaphthalene manufacturer
Ladleadd
Neshiel Agrochem Private Limited
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 2-Methoxynaphthalene 99%View Details
2-Methoxynaphthalene 99%View Details -
 2-Methoxynaphthalene 98%View Details
2-Methoxynaphthalene 98%View Details -
 2-Methoxynaphthalene 98%View Details
2-Methoxynaphthalene 98%View Details -
 2-Methoxynaphthalene 98%View Details
2-Methoxynaphthalene 98%View Details -
 Methyl 2-Naphthyl Ether pure CAS 93-04-9View Details
Methyl 2-Naphthyl Ether pure CAS 93-04-9View Details
93-04-9 -
 Yara Yara, For PerfumeryView Details
Yara Yara, For PerfumeryView Details
93-04-9 -
 99% Beta Naphthyl Methyl Ether Yara Yara, For PerfumeryView Details
99% Beta Naphthyl Methyl Ether Yara Yara, For PerfumeryView Details
93-04-9 -
 99.9% Yara Yara 2- methoxy Naphthalene, For Perfumery, Packaging Size: 25 KGView Details
99.9% Yara Yara 2- methoxy Naphthalene, For Perfumery, Packaging Size: 25 KGView Details
93-04-9
