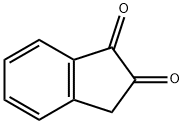
2-Indanone
Synonym(s):β-Hydrindone
- CAS NO.:615-13-4
- Empirical Formula: C9H8O
- Molecular Weight: 132.16
- MDL number: MFCD00003792
- EINECS: 210-410-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-08-24 19:19:26
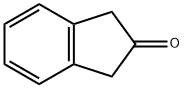
What is 2-Indanone?
Chemical properties
Wet crystalline aggregates or needle-like crystals (formed by ethanol or ether) evaporate when in contact with water vapor. These crystals have a melting point range of 58-61 ℃ and a flash point of 100 ℃. Indene compounds are commonly found in numerous natural products, exhibiting diverse physiological activities and serving as crucial intermediates in organic synthesis processes.
The Uses of 2-Indanone
2-Indanone undergoes TiCl4-Mg mediated coupling with CHBr3 to yield dibromomethyl carbinol. It reacts with 5,5-dimethyl-3-pyrazolidinone to yield 5,5-dimethyl-2-(1H-indenyl-2)-3-pyrazolidinone. On photolysis by 266-nm one-photon excitation yields o-xylylene. It was used as starting reagent in the synthesis of indene-fused porphyrins.
Preparation
2-Indanone is prepared by using acetic acid as solvent, acetic anhydride as catalyst and through hydrogen peroxide oxidation into 1, 2-indenediol, which reacted with dilute sulfuric acid solution in order to obtain crude 2-indanone. Finally, vacuum sublimation to obtain 2-indanone with high-purity, the total yield is 89%.
What are the applications of Application
2-Indanone is mentioned mainly for the completion and illustration of the series in which dpila- and be{u-Hydrindone have been emphasized as having potential interest in the perfume industry. It is the author's impression that the two materials are not used in perfumes or flavors.
Definition
ChEBI: 2-Indanone is an indanone with an oxo substituent at position 2. It is a metabolite of indane. It has a role as a xenobiotic metabolite. 2-Indanone is an intermediate for the preparation of aprindine hydrochloride and ceforanide. It is an important intermediate in organic synthesis.
Synthesis Reference(s)
Chemistry Letters, 11, p. 325, 1982
Organic Syntheses, Coll. Vol. 5, p. 647, 1973
Tetrahedron Letters, 15, p. 3789, 1974 DOI: 10.1016/S0040-4039(01)92010-6
General Description
2-Indanone undergoes TiCl4-Mg mediated coupling with CHBr3 to yield dibromomethyl carbinol. It reacts with 5,5-dimethyl-3-pyrazolidinone to yield 5,5-dimethyl-2-(1H-indenyl-2)-3-pyrazolidinone. 2-Indanone on photolysis by 266-nm one-photon excitation yields o-xylylene.
Properties of 2-Indanone
| Melting point: | 51-54 °C (lit.) |
| Boiling point: | 218°C |
| Density | 1.0712 |
| refractive index | 1.5380 (estimate) |
| Flash point: | 212 °F |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Ethyl Acetate (Slightly) |
| form | Crystals or Powder |
| color | Light yellow to yellow-brown |
| Specific Gravity | 1.07 (liquid) |
| Odor | moderately tenacious odor |
| Odor Threshold | 0.00036ppm |
| Water Solubility | insoluble |
| Sensitive | Hygroscopic |
| BRN | 636550 |
| CAS DataBase Reference | 615-13-4(CAS DataBase Reference) |
| NIST Chemistry Reference | 2H-inden-2-one, 1,3-dihydro-(615-13-4) |
| EPA Substance Registry System | 2-Indanone (615-13-4) |
Safety information for 2-Indanone
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2-Indanone
| InChIKey | UMJJFEIKYGFCAT-UHFFFAOYSA-N |
Abamectin manufacturer
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Mefenamic Acid IP/BP/EP/USP Diclofenac Sodium IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

![N-BOC-1-[4-SPIRO-PIPERIDINE]-2-INDANONE](https://img.chemicalbook.in/StructureFile/ChemBookStructure5/GIF/CB0342508.gif)

You may like
-
 2-Indanone, 98% CAS 615-13-4View Details
2-Indanone, 98% CAS 615-13-4View Details
615-13-4 -
 2-Indanone CAS 615-13-4View Details
2-Indanone CAS 615-13-4View Details
615-13-4 -
 2-Indanone CAS 615-13-4View Details
2-Indanone CAS 615-13-4View Details
615-13-4 -
 2-Indanone CAS 615-13-4View Details
2-Indanone CAS 615-13-4View Details
615-13-4 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4