2-Formyl-3,4-dihydro-2H-pyran
- CAS NO.:100-73-2
- Empirical Formula: C6H8O2
- Molecular Weight: 112.13
- MDL number: MFCD00059170
- EINECS: 202-884-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-25 18:00:57
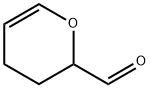
What is 2-Formyl-3,4-dihydro-2H-pyran?
Chemical properties
Colorless viscous liquid
The Uses of 2-Formyl-3,4-dihydro-2H-pyran
NSC 95413 is used as a reagent in chemical processes.
What are the applications of Application
3,4-dihydro-2H-pyran-2-carbaldehyde is A cyclic esther aldehyde compound used for biochemical research.
Synthesis Reference(s)
Journal of the American Chemical Society, 92, p. 3126, 1970 DOI: 10.1021/ja00713a034
General Description
A colorless or yellow liquid with a pungent disagreeable odor. Flash point 118°F. Density 1.077 g / cm3 (8.96 lb / gal). May be irritating to the eyes and mucous membranes; long-term exposure may result in adverse health effects. Vapors denser than air. Used to make other chemicals, plastics.
Air & Water Reactions
Flammable. Soluble in water.
Reactivity Profile
Aldehydes are frequently involved in self-condensation or polymerization reactions. These reactions are exothermic; they are often catalyzed by acid. Aldehydes are readily oxidized to give carboxylic acids. Flammable and/or toxic gases are generated by the combination of aldehydes with azo, diazo compounds, dithiocarbamates, nitrides, and strong reducing agents. Aldehydes can react with air to give first peroxo acids, and ultimately carboxylic acids. These autoxidation reactions are activated by light, catalyzed by salts of transition metals, and are autocatalytic (catalyzed by the products of the reaction). The addition of stabilizers (antioxidants) to shipments of aldehydes retards autoxidation.
Health Hazard
May cause toxic effects if inhaled or absorbed through skin. Inhalation or contact with material may irritate or burn skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. May polymerize explosively when heated or involved in a fire. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Safety Profile
Mildly toxic by ingestion. A skin and severe eye irritant. A flammable liquid when exposed to heat, flame, or powerful oxidizing agents. To fight fire, use alcohol foam and multipurpose dry chemical. When heated to decomposition it emits acrid smoke and fumes
Properties of 2-Formyl-3,4-dihydro-2H-pyran
| Melting point: | -99.9°C |
| Boiling point: | 146 °C(lit.) |
| Density | 1.08 g/mL at 25 °C |
| refractive index | n20/D 1.466(lit.) |
| Flash point: | 54 °C |
| CAS DataBase Reference | 100-73-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Acrolein dimer(100-73-2) |
| EPA Substance Registry System | 2H-Pyran-2-carboxaldehyde, 3,4-dihydro- (100-73-2) |
Safety information for 2-Formyl-3,4-dihydro-2H-pyran
Computed Descriptors for 2-Formyl-3,4-dihydro-2H-pyran
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran



You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4