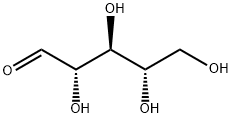
2-Deoxy-D-ribose
Synonym(s):2-Deoxy-D -arabinose;2-Deoxy-D -erythropentose;Thyminose
- CAS NO.:533-67-5
- Empirical Formula: C5H10O4
- Molecular Weight: 134.13
- MDL number: MFCD00135904
- EINECS: 208-573-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 16:58:18
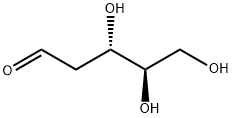
What is 2-Deoxy-D-ribose?
Description
2-deoxy-D-Ribose is a reducing sugar formed as a degradation product during metabolism of thymidine by thymidine phosphorylase. It increases levels of reactive oxygen species (ROS) in HL-60 human leukemia cells when used at a concentration of 15 mM. 2-deoxy-D-Ribose (10 μM) induces tubulogenesis and migration of bovine aortic endothelial (BAE) cells. Topical administration of 2-deoxy-D-ribose increases blood vessel formation and accelerates wound healing in a rat full-thickness cutaneous wound model.
Chemical properties
White powder
The Uses of 2-Deoxy-D-ribose
2-Deoxy-D-ribose is used as a precursor to deoxyribonucleic acid. It induces apoptosis by inhibiting the synthesis and increasing the efflux of glutathione. Further, it is used in the synthesis of optically active dipyrrolyl alkanols from pyrroles on the surface of montmorillonite KSF clay.
Definition
ChEBI: 2-deoxy-D-ribose is a deoxypentose that is D-ribose in which the hydroxy group at position C-2 is replaced by hydrogen. It has a role as a human metabolite, a Saccharomyces cerevisiae metabolite and a mouse metabolite. It is functionally related to a D-ribose.
Purification Methods
Dissolve 2-deoxy--D-ribose in a little H2O, evaporate to a syrup (in a vacuum), and seed to crystallise. Triturate the crystals with a little EtOAc containing 5% MeOH, decant and dry in vacuum over P2O5. It is best purified via the anilide which separates from a mixture of the ribose (100-125g) in MeOH (100mL) and redistilled aniline (40mL) in a few minutes. After standing for 20hours at room temperature, it is cooled to 0o, filtered, washed with 50% aqueous MeOH and Et2O followed by recrystallisation from ethylene glycol monomethyl ether. The anilide has m 172-173o, [ ] D 25 +46o (equilibrium in pyridine). The anilide (5g), benzaldehyde (5mL) and benzoic acid (0.5g) in H2O (150mL) are shaken mechanically for 2024hours. The aqueous phase is extracted with Et2O (3x), decolourised with a little charcoal and evaporated in a vacuum to a syrup. This is dried over P2O5 in high vacuum. The syrupy sugar weighs 3.1g and crystallises in a few days, but more rapidly on seeding. Triturate it with a little EtOAc containing 5% MeOH, decant and dry it over P2O5. At this stage it has m 78-82o, [ ] D 25 -57o (c 1, H2O final). This is a mixture of and anomers. Pure -anomer is obtained by recrystallisation from EtOAc The -anomer when recrystallised from EtOAc and isoPrOH has m 96-98o, [ ] D 25 -55o (c 0.5, H2O final). [Sowden Biochemical Preparations 5 75 1957.] The mutarotation is as follows: [] D 20.5 +96.3o(0minutes), -76o(33minutes), -56o (24hours) (c 5.8 MeOH). It is moderately hygroscopic and should be kept in a well stoppered bottle. It also crystallises from diethyl ether. [Deriaz et al. J Chem Soc 1879 1949, Beilstein 1 IV 4181, Hauske & Rapoport J Org Chem 4 4 2472 1979.]
Properties of 2-Deoxy-D-ribose
| Melting point: | 89-90 °C(lit.) |
| Boiling point: | 167.23°C (rough estimate) |
| alpha | -57 º (c=1, H2O, 24hr) |
| Density | 1.0590 (rough estimate) |
| refractive index | -56 ° (C=1, H2O) |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | DMSO (Slightly), Methanol (Slightly), Water (Slightly, Sonicated) |
| form | Crystalline Powder |
| pka | 12.61(at 25℃) |
| color | White to slightly yellow |
| optical activity | [α]22/D 59°, c = 1 in H2O |
| Water Solubility | soluble |
| Sensitive | Hygroscopic |
| Merck | 14,2908 |
| BRN | 1721978 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 533-67-5(CAS DataBase Reference) |
| NIST Chemistry Reference | D-Erythro-pentose, 2-deoxy-(533-67-5) |
| EPA Substance Registry System | D-erythro-Pentose, 2-deoxy- (533-67-5) |
Safety information for 2-Deoxy-D-ribose
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H332:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2-Deoxy-D-ribose
| InChIKey | ASJSAQIRZKANQN-CRCLSJGQSA-N |
2-Deoxy-D-ribose manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 533-87-5 2-Deoxy-D-Ribose 98%View Details
533-87-5 2-Deoxy-D-Ribose 98%View Details
533-87-5 -
 533-67-5 99%View Details
533-67-5 99%View Details
533-67-5 -
 2-Deoxy-D-ribose CAS 533-67-5View Details
2-Deoxy-D-ribose CAS 533-67-5View Details
533-67-5 -
 2-Deoxy D-ribose CAS 533-67-5View Details
2-Deoxy D-ribose CAS 533-67-5View Details
533-67-5 -
 2-Deoxy-D-ribose CAS 533-67-5View Details
2-Deoxy-D-ribose CAS 533-67-5View Details
533-67-5 -
 2-Deoxy-D-ribose CAS 533-67-5View Details
2-Deoxy-D-ribose CAS 533-67-5View Details
533-67-5 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
