2-Chloroadenosine
Synonym(s):2-CADO;6-Amino-2-chloropurine riboside
- CAS NO.:146-77-0
- Empirical Formula: C10H12ClN5O4
- Molecular Weight: 301.69
- MDL number: MFCD00005734
- EINECS: 205-678-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
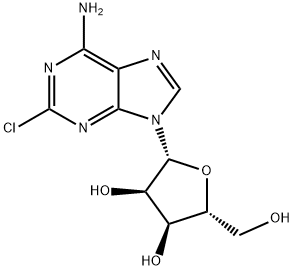
What is 2-Chloroadenosine?
Chemical properties
White to Off-White Crystalline Sold
The Uses of 2-Chloroadenosine
Selective A1 adenosine receptor agonist. Since 2-chloroadenosine is not deaminated by adenosine deaminase and is less rapidly taken up by the brain than adenosine, 2-chloroadenosine led to more marked increase in CBF than adenosine.
The Uses of 2-Chloroadenosine
A selective A1-adenosine receptor agonist. Induces apoptosis
The Uses of 2-Chloroadenosine
2-Chloroadenosine is used as a selective A1-adenosine receptor agonist, and also Induces apoptosis.
What are the applications of Application
2-Chloroadenosine is an Adenosine A receptor agonist
Definition
A metabolically stable analog of adenosine which acts as an adenosine receptor agonist. The compound has a potent effect on the peripheral and central nervous system.
Biological Activity
Metabolically stable analog of adenosine that behaves as an adenosine receptor agonist (K i values are 300, 80 and 1900 nM for A 1 , A 2A and A 3 receptors respectively). Anticonvulsive in vivo .
Biochem/physiol Actions
2-Chloroadenosine is an analog of adenosine. It is an adenosine A1 receptor agonist. It might possess anti-oxidant property.
Pharmacology
2-chloroadenosine (2-CAdo) is an adenosine deaminase-resistant analogue of adenosine, widely used as an adenosine receptor agonist. This compound has been shown to induce apoptosis in several cell types either via activation of adenosine receptors or via intracellular metabolism. However, the molecular mechanisms of 2-CAdo-induced apoptosis are unclear. Here, we analyzed the effects of 2-CAdo in the leukemia cell line EHEB. 2-CAdo was found to induce apoptosis in EHEB cells, as shown by caspase-3 activation, DNA fragmentation, poly(ADP-ribose) polymerase (PARP) cleavage and phosphatidylserine exposure. Cytotoxicity of 2-CAdo was completely suppressed by 5-iodotubercidin, an adenosine kinase inhibitor, indicating that apoptosis induced by 2-CAdo was the result of its intracellular metabolism. Accordingly, we found that 2-CAdo was efficiently converted into 2-chloroATP. In parallel, a decrease of intracellular ATP concentration as well as a general inhibition of macromolecular synthesis, involving DNA, RNA and protein synthesis, was observed. Moreover, 2-CAdo induced cytochrome c release into the cytosol, indicating activation of the intrinsic pathway of apoptosis. This was found associated with a decline in Mcl-1 protein level and p53-independent. Inhibition of AMP deaminase by coformycin markedly prevented ATP depletion, and also significantly reduced 2-CAdo cytotoxicity and caspase-3 activation. In conclusion, our data show that intracellular metabolism of 2-CAdo can lead to activation of the intrinsic pathway of apoptosis and that ATP depletion, in addition to the accumulation of the triphosphate analogue, contributes to 2-CAdo-induced apoptosis.
storage
Store at +4°C
Purification Methods
Purify 2-chloroadenosine by recrystallisation from H2O (~1% in cold), and it has max at 264 nm (pH 1 and 7) and 265 nm (pH 13) in H2O. [Brown & Weliky J Org Chem 23 125 1958, Schaeffer & Thomas J Am Chem Soc 80 3738 1958, IR: Davoll & Lewy J Am Chem Soc 74 1563 1952, Beilstein 26 III/IV 3725.]
Properties of 2-Chloroadenosine
| Melting point: | 162 °C |
| Boiling point: | 591.8±60.0 °C(Predicted) |
| Density | 1.8359 (rough estimate) |
| refractive index | -50 ° (C=0.1, H2O) |
| storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C |
| solubility | H2O: 10 mg/mL, clear, colorless |
| form | Solid |
| pka | 13.05±0.70(Predicted) |
| color | White to Off-White |
| Water Solubility | Soluble in water. |
| BRN | 43957 |
| CAS DataBase Reference | 146-77-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Adenosine, 2-chloro-(146-77-0) |
Safety information for 2-Chloroadenosine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H332:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2-Chloroadenosine
| InChIKey | BIXYYZIIJIXVFW-UUOKFMHZSA-N |
2-Chloroadenosine manufacturer
JSK Chemicals
New Products
2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-Pyridineacetonitrile, α-hydroxy- 3-Iodophenylacetic acid 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 2-Hexanone, 98% Sodium molybdate dihydrate,98% 2,3-Dimethylphenol, 98% 4,4'-Oxydianiline Isoquinoline, 96% 2-Amino-4,6-difluorobenzoic acid, 95% (R)-2-Methylpyrolidine-2-carboxylic acid (De Mepro) Ramipril Sacubitril- Valsartan Boc-his(trt)-OH Fmoc-L-Glu-OtBu Boc-L-Tyr(tBu)-OH N N Dimethylformamide Dimethyl Acetal (Dmf Dma) Semi carbazide Hydrochloride 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] Trans-4-Aminocyclohexanol [4tac] 2-[1-(Mercaptomethyl)Cyclopropyl]Acetic Acid 2-Chloromethyl-4-methyl-quinazolineRelated products of tetrahydrofuran

![2-CHLOROADENOSINE, [8-14C]-](https://img.chemicalbook.in/StructureFile/ChemBookStructure4/GIF/CB4184665.gif)
![2-CHLOROADENOSINE, [8-3H]-](https://img.chemicalbook.in/StructureFile/ChemBookStructure4/GIF/CB2116558.gif)
![(+)-5'-O-[[(S)-2-Amino-1-oxopropyl]aminosulfonyl]-2-chloroadenosine](https://img.chemicalbook.in/CAS/GIF/91432-48-3.gif)
You may like
-
 146-77-0 (3R, 4S, 5R)-2-(6-amino-2-chloro-9H-purin-9yl)-5-(hydroxymethyl) tetrahydrofuran-3, 4-diol 98%View Details
146-77-0 (3R, 4S, 5R)-2-(6-amino-2-chloro-9H-purin-9yl)-5-(hydroxymethyl) tetrahydrofuran-3, 4-diol 98%View Details
146-77-0 -
 2-Chloroadenosine 98%View Details
2-Chloroadenosine 98%View Details -
 2-Chloroadenosine 146-77-0 98%View Details
2-Chloroadenosine 146-77-0 98%View Details
146-77-0 -
 146-77-0 2-Chloroadenosine 98%View Details
146-77-0 2-Chloroadenosine 98%View Details
146-77-0 -
 2-Chloroadenosine,98% 146-77-0 99%View Details
2-Chloroadenosine,98% 146-77-0 99%View Details
146-77-0 -
 2-Chloroadenosine CAS 146-77-0View Details
2-Chloroadenosine CAS 146-77-0View Details
146-77-0 -
 2-Chloroadenosine CAS 146-77-0View Details
2-Chloroadenosine CAS 146-77-0View Details
146-77-0 -
 15761-38-3 N-Boc-L-Alanine >98%View Details
15761-38-3 N-Boc-L-Alanine >98%View Details
15761-38-3
