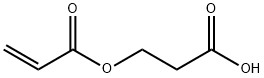
2-Carboxyethyl acrylate
Synonym(s):2-Carboxyethyl acrylate;3-(Acryloyloxy)propionic acid;3-Acryloyloxypropanoic acid;Acrylic acid 2-carboxyethyl ester;Acrylic acid dimer
- CAS NO.:24615-84-7
- Empirical Formula: C6H8O4
- Molecular Weight: 144.13
- MDL number: MFCD00040709
- EINECS: 246-359-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
What is 2-Carboxyethyl acrylate?
Description
2-Carboxyethyl acrylate is a highly versatile monomer extensively used to produce diverse polymers. It can be polymerized in solution or emulsion, producing vinyl-acrylic, acrylic, or styrenic-acrylic polymers with greater flexibility due to lower glass transition of itshomopolymer (<30C) and improved adhesion. It finds applications in various polymer production processes, including those geared towards optical applications like optical fibers, optical waveguides, and optical lenses. Moreover, 2-Carboxyethyl acrylate is instrumental in synthesizing polymers used in energy storage applications, such as fuel cells, batteries, and supercapacitors. 2-carboxyethyl acrylate could be used as a new monomer in the gel casting of alumina. This low-toxic, water-soluble monomer modifies the ceramic suspensions' rheological properties and minimizes the oxygen inhibition's negative effect [1-3].
Chemical properties
Colorless to light yellow viscid liquid
The Uses of 2-Carboxyethyl acrylate
2-Carboxyethyl Acrylate is used in the preparation of DNase enzyme derivatives that act as potent preventative material of bacterial adhesion and biofilm formation in biomaterials.
What are the applications of Application
2-Carboxyethyl acrylate is used to produce vinyl-acrylic, acrylic, or styrenic-acrylic polymers
What are the applications of Application
2-Carboxyethyl acrylate can be polymerized in solution or emulsion to produce acrylic, vinyl acrylic, or styrene acrylic polymers, which are distinguished by their low glass transition temperatures (<30°C) as homopolymers. Greater elasticity, as well as improved adhesion.
Preparation
Synthesis of 1-ethoxyethyl acrylate (EEA) and protected 2-carboxyethyl acrylate (proCEA)
EEA and proCEA were synthesized following a previously published procedure and distilled prior to use. For the synthesis of proCEA (Figure 1), phosphoric acid (109 mg, 1.11 mmol) was weighed into a dry round bottom flask in a glovebox and then taken outside the glovebox, taking care that the phosphoric acid stayed dry. 2-Carboxyethyl acrylate (80 g, 555 mmol) and ethyl vinyl ether (48 g, 666 mmol) were added and the reaction was stirred for two days at room temperature. Hydrotalcite (Mg6Al2(OH)16CO3·4H2O, ~1 g) was added, stirred for one hour and filtered off. Excess ethyl vinyl ether was removed under reduced pressure and the product was distilled under reduced pressure (80 °C, 1.3 mbar).
Figure 1 Synthesis of proCEA
Hazard
A severe skin irritant.
References
[1] Emilia Pietrzak, Mikolaj Szafran, Paulina Wiecinska. “2-carboxyethyl acrylate as a new monomer preventing negative effect of oxygen inhibition in gelcasting of alumina.” Ceramics International 42 12 (2016): Pages 13682-13688.
[2] Naveed Ullah . “Coupling of carboxymethyl starch with 2-carboxyethyl acrylate: A new sorbent for the wastewater remediation of methylene blue.” Environmental Research 219 (2023): Article 115091.
[3] Amit K. Tripathi, Donald C. Sundberg*, Jenna Vossoughi. “Partitioning of 2-Carboxyethyl Acrylate between Water and Vinyl Monomer Phases Applied to Emulsion Polymerization: Comparisons with Hydroxy Acrylate and Other Vinyl Acid Functional Monomers.” Industrial Engineering Chemistry Research 54 9 (2015): 2447–2452.
Properties of 2-Carboxyethyl acrylate
| Boiling point: | 103 °C/19 mmHg (lit.) |
| Density | 1.214 g/mL at 25 °C (lit.) |
| refractive index | n |
| Flash point: | >230 °F |
| storage temp. | 2-8°C |
| solubility | H2O: soluble |
| pka | 3.95±0.10(Predicted) |
| PH | 2.95 (10wt. % in H2O) |
| Merck | 13,132 |
| InChI | InChI=1S/C6H8O4/c1-2-6(9)10-4-3-5(7)8/h2H,1,3-4H2,(H,7,8) |
| EPA Substance Registry System | Hydracrylic acid acrylate (24615-84-7) |
Safety information for 2-Carboxyethyl acrylate
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P363:Wash contaminated clothing before reuse. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2-Carboxyethyl acrylate
| InChIKey | CYUZOYPRAQASLN-UHFFFAOYSA-N |
| SMILES | C(OCCC(O)=O)(=O)C=C |
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran



You may like
-
 2-Carboxyethyl acrylate CAS 24615-84-7View Details
2-Carboxyethyl acrylate CAS 24615-84-7View Details
24615-84-7 -
 17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 -
 131987-69-4 98+View Details
131987-69-4 98+View Details
131987-69-4 -
 Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
67967-07-1 -
 764-60-3 2-Hexyn-1-ol 98+View Details
764-60-3 2-Hexyn-1-ol 98+View Details
764-60-3 -
 2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
5968-21-8 -
 3-Iodophenylacetic acid 1878-69-9 98+View Details
3-Iodophenylacetic acid 1878-69-9 98+View Details
1878-69-9 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
