16-HYDROXYHEXADECANOIC ACID
Synonym(s):16-Hydroxypalmitic acid;Juniperic acid
- CAS NO.:506-13-8
- Empirical Formula: C16H32O3
- Molecular Weight: 272.42
- MDL number: MFCD00002750
- EINECS: 208-028-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
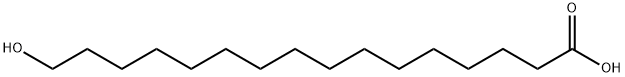
What is 16-HYDROXYHEXADECANOIC ACID?
Description
16-
Chemical properties
white crystalline solid
The Uses of 16-HYDROXYHEXADECANOIC ACID
16-Hydroxyhexadecanoic acid was used in the synthesis of dihydroxypalmitic acids. It was also used to induce the expression of two GRP genes of Arabidopsis thaliana, AtGRP5 and AtGRP23.
Definition
ChEBI: 16-hydroxyhexadecanoic acid is an omega-hydroxy-long-chain fatty acid that is hexadecanoic acid (also known as palmitic acid) which is substituted at position 16 by a hydroxy group. It is a key monomer of cutin in the plant cuticle. It has a role as a plant metabolite. It is an omega-hydroxy-long-chain fatty acid and a hydroxypalmitic acid. It is a conjugate acid of a 16-hydroxyhexadecanoate.
What are the applications of Application
16-Hydroxyhexadecanoic acid can be used to:
(1) Oxidative reactions: Cell lysates and intact cells of Escherichia coli JM101 recombinant, which synthesises the cyp102 gene for cytochrome P450BM-3 monooxygenase, biotransform 16-hydroxyhexa-decanoic acid into 1, 16-hexadecanedioic acid. a small amount of intermediate product accumulation accompanies the oxidation of 16-hydroxyhexadecanoic acid. hydroxyhexadecanoic acid oxidation is accompanied by the accumulation of a small amount of intermediate products. The by-products also include 13.16-dihydroxyhexadecanoic acid and 12,16-dihydroxyhexadecanoic acid[1].
(2) 16-hydroxyhexadecanoic acid ethyl ester was synthesised from 16-hexadecanolactone by an intramolecular ester exchange reaction with PEG-lipase[2].
(3) Lactonisation reaction: It has been demonstrated that Mucor javanicus L46 and Mucor miehei catalyse the lactonisation reaction of 15-hydroxypentadecanoic and 16- hydroxyhexadecanoic acids to appropriate macrocyclic mono- and oligolactones[3].
References
[1] S. SCHNEIDER. Production Of Alkanedioic Acids By Cytochrome P450Bm-3 Monooxygenase: Oxidation Of 16-Hydroxyhexadecanoic Acid To Hexadecane-1, 16-Dioic Acid[J]. Biocatalysis and Biotransformation, 1999. DOI:10.3109/10242429909040113.
[2] YOH KODERA . Lactone synthesis from 16-hydroxyhexadecanoic acid ethyl ester in organic solvents catalyzed with polyethylene glycol-modified lipase[J]. Journal of biotechnology, 1993. DOI:10.1016/0168-1656(93)90162-G.
[3] U. ANTCZAK . Enzymatic lactonization of 15-hydroxypentadecanoic and 16-hydroxyhexadecanoic acids to macrocyclic lactones[J]. Enzyme and Microbial Technology, 1991. DOI:10.1016/0141-0229(91)90095-R.
Properties of 16-HYDROXYHEXADECANOIC ACID
| Melting point: | 94-98 °C(lit.) |
| Boiling point: | 414.4±18.0 °C(Predicted) |
| Density | 0.956±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMF: 20 mg/ml; DMSO: 20 mg/ml; DMSO:PBS(pH 7.2) (1:2): 0.33 mg/ml; Ethanol: 2.5 mg/ml |
| pka | 4.78±0.10(Predicted) |
| form | Crystalline Powder |
| color | White |
| BRN | 1783998 |
| InChI | InChI=1S/C16H32O3/c17-15-13-11-9-7-5-3-1-2-4-6-8-10-12-14-16(18)19/h17H,1-15H2,(H,18,19) |
| CAS DataBase Reference | 506-13-8(CAS DataBase Reference) |
| EPA Substance Registry System | 16-Hydroxypalmitic acid (506-13-8) |
Safety information for 16-HYDROXYHEXADECANOIC ACID
Computed Descriptors for 16-HYDROXYHEXADECANOIC ACID
| InChIKey | UGAGPNKCDRTDHP-UHFFFAOYSA-N |
| SMILES | C(O)(=O)CCCCCCCCCCCCCCCO |
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

You may like
-
 16-Hydroxyhexadecanoic Acid CAS 506-13-8View Details
16-Hydroxyhexadecanoic Acid CAS 506-13-8View Details
506-13-8 -
 16-Hydroxyhexadecanoic acid CAS 506-13-8View Details
16-Hydroxyhexadecanoic acid CAS 506-13-8View Details
506-13-8 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4