1,5-Naphthalenediamine
Synonym(s):1,5-DAN;1,5-Naphthalenediamine;NSC 401110
- CAS NO.:2243-62-1
- Empirical Formula: C10H10N2
- Molecular Weight: 158.2
- MDL number: MFCD00004029
- EINECS: 218-817-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
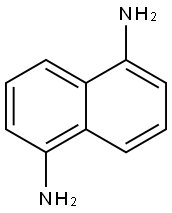
What is 1,5-Naphthalenediamine?
Chemical properties
GREY TO DARK BROWN POWDER. 1,5-Naphthalenediamine [2243-62-1]. 1,5-diaminonaphthalene, Alphamin, C10H10N2, Mr 158.2: oxidation of 97 with iron (III) chloride in water produces a blue-violet color. Treatment with boiling aqueous sodium bisulfite followed by addition of alkali gives a mixture of 1-amino-5-hydroxynaphthalene and 1,5-dihydroxynaphthalene. Sulfonation (5 % oleum, 100℃) gives 1,5-diaminonaphthalene2-sulfonic acid, and nitration in acetic acid produces 2,4,6,8-tetranitro-1,5-diaminonaphthalene. Coupling with diazo compounds takes place in the 2-position; reduction of the resulting azo compound with SnCl2– HCl produces 1,2,5- triaminonaphthalene.
The Uses of 1,5-Naphthalenediamine
1,5-naphthalenediamine be used as intermediates for the synthesis of materials.
The Uses of 1,5-Naphthalenediamine
Aromatic amines likely to have high carcinogenic potency. QSARs of aromatic amines.
The Uses of 1,5-Naphthalenediamine
1,5-Naphthalenediamineis an important raw material and intermediate used in organic synthesis, pharmaceuticals, agrochemicals and dyestuffs. The reducing properties of 1,5-diaminonaphthalene (1,5-DAN) as a MALDI matrix ( matrix-assisted laser desorption ionization) to amino acid sequencing and disulfide bond mapping of human urotensin II possessing one disulfide bond, and human guanylin possessing two disulfide bonds.
Definition
ChEBI: A naphthalenediamine compound having amino substituents in the 1- and 5-positions.
Production Methods
1-Nitronaphthalene can be nitrated further to give a 40 : 60 mixture of 1,5- and 1,8-dinitronaphthalenes. Similar results are obtained by direct nitration of naphthalene with H2SO4– HNO3 under careful control of temperature over the range 40 – 80℃. Although separation of the isomers by fractional crystallization or solvent extraction is usually carried out at this stage, the mixed isomers can also be reduced and the resulting diamines separated. Reduction of the dinitronaphthalenes is achieved by treatment of a nonaqueous solution with iron or hydrogen in the presence of a catalyst. An alternative process for 1,5-naphthalenediamine involves amination of 1,5-dihydroxynaphthalene with ammonia and ammonium bisulfite. Although less efficient on a stage basis it offers an economical alternative to nitration and reduction if the 1,8-naphthalenediamine is not also required.

General Description
Colorless to pale purple crystals or lavender powder.
Air & Water Reactions
1,5-Naphthalenediamine is sensitive to prolonged exposure to air. Insoluble in water.
Reactivity Profile
1,5-Naphthalenediamine neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides.
Hazard
Questionable carcinogen.
Fire Hazard
Flash point data for 1,5-Naphthalenediamine are not available. 1,5-Naphthalenediamine is probably combustible.
Safety Profile
Suspected carcinogen with experimental carcinogenic, neoplastigenic, tumorigenic data. Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx.
Purification Methods
Recrystallise the amino-naphthalene from boiling H2O, but this is wasteful due to poor solubility. Boil it in chlorobenzene (charcoal), filter hot and cool the filtrate (preferably under N2). This gives colourless crystals. Dry it in a vacuum till free from chlorobenzene (odour), and store it in sealed ampoules under N2 away from light. [Beilstein 13 IV 340.]
Properties of 1,5-Naphthalenediamine
| Melting point: | 185-187 °C(lit.) |
| Boiling point: | 200-210°C 5mm |
| Density | 1.4 |
| vapor pressure | 0Pa at 20℃ |
| refractive index | 1.6441 (estimate) |
| Flash point: | 200-210°C/5mm |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | 0.04g/l |
| form | Powder |
| pka | 4.59±0.10(Predicted) |
| color | Gray to dark brown |
| Water Solubility | <0.1 g/100 mL at 20.5 ºC |
| BRN | 907947 |
| CAS DataBase Reference | 2243-62-1(CAS DataBase Reference) |
| IARC | 3 (Vol. 27, Sup 7) 1987 |
| NIST Chemistry Reference | 1,5-Naphthalenediamine(2243-62-1) |
| EPA Substance Registry System | 1,5-Naphthalenediamine (2243-62-1) |
Safety information for 1,5-Naphthalenediamine
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H351:Carcinogenicity H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P391:Collect spillage. Hazardous to the aquatic environment P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. |
Computed Descriptors for 1,5-Naphthalenediamine
1,5-Naphthalenediamine manufacturer
LANXESS India Pvt. Ltd.
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 2243-62-1 1,5-Diaminonaphthalene 99%View Details
2243-62-1 1,5-Diaminonaphthalene 99%View Details
2243-62-1 -
 1,5-Naphthalenediamine 98%View Details
1,5-Naphthalenediamine 98%View Details -
 1,5-Naphthalenediamine 98%View Details
1,5-Naphthalenediamine 98%View Details
2243-62-1 -
 1,5-Diaminonaphthalene (purified by sublimation) CAS 2243-62-1View Details
1,5-Diaminonaphthalene (purified by sublimation) CAS 2243-62-1View Details
2243-62-1 -
 1,5-Diaminonaphthalene CAS 2243-62-1View Details
1,5-Diaminonaphthalene CAS 2243-62-1View Details
2243-62-1 -
 1,5-Diaminonaphthalene CAS 2243-62-1View Details
1,5-Diaminonaphthalene CAS 2243-62-1View Details
2243-62-1 -
 1,5-Diaminonaphthalene CAS 2243-62-1View Details
1,5-Diaminonaphthalene CAS 2243-62-1View Details
2243-62-1 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1