1-BUTENE
- CAS NO.:106-98-9
- Empirical Formula: C4H8
- Molecular Weight: 56.11
- MDL number: MFCD00009383
- EINECS: 203-449-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
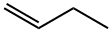
What is 1-BUTENE?
Description
1-Butene is a colourless, stable but polymerises exothermically, extremely flammable liquefied gas with an aromatic odour. It is insoluble in water and is one of the isomers of butane. 1-Butene readily forms explosive mixtures with air. It is incompatible with strong oxidising agents, halogens, halogen acids, metal salts, boron trifluoride, fluorine, and nitrogen oxides. 1-Butene of high purity is made by cracking naphtha and separating it from other products by an extra-high-purity distillation column. It is an important organic compound in the production of several industrial materials – for instance, linear low-density polyethylene (LLDPE), a more flexible and resilient polyethylene, and a range of polypropylene resins – and in the production of polybutene, butylene oxide, and the C4 solvents secondary butyl alcohol (SBA) and MEK. The vapour of 1-butene is heavier than air and may travel long distances to an ignition source and flash back.
Chemical properties
1-Butene is a colorless, extremely flammable liquefi ed gas with an aromatic odor. It is insol uble in water and is an isomer of butane. It is highly flammable and readily forms explo sive mixtures with air. 1-Butene of high purity is made by cracking naphtha and separating it from other products by an extra-high purity distillation column. However, 1-butene is incompatible with metal salts, fl uorine, nitrogen oxides, boron trifl uoride, halogen acids, halogens, and strong oxidizing agents. It is an important organic compound in the produc tion of several industrial materials, i.e., linear low density polyethylene (LLDPE), a more fl exible and resilient polyethylene, a range of polypropylene resins, and in the production of polybutene, butylene oxide and in the C4 solvents, secondary butyl alcohol (SBA) and methyl ethyl ketone (MEK). The vapor of 1-butene is heavier than air and may travel long distances to an ignition source and fl ash back.
Physical properties
Butenes or butylenes are hydrocarbon alkenes that exist as four different isomers. Each isomer is a flammable gas at normal room temperature and one atmosphere pressure, but their boiling points indicate that butenes can be condensed at low ambient temperatures and/or increase pressure similar to propane and butane. The “2” designation in the names indicates the position of the double bond. The cis and trans labels indicate geometric isomerism. Geometric isomers are molecules that have similar atoms and bonds but different spatial arrangement of atoms. The structures indicate that three of the butenes are normal butenes, n-butenes, but that methylpropene is branched. Methylpropene is also called isobutene or isobutylene. Isobutenes are more reactive than n-butenes, and reaction mechanisms involving isobutenes differ from those of normal butenes.
The Uses of 1-BUTENE
Butenes are used extensively in gasoline production to produce high-octane gasoline compounds.Another large use of normal butenes in the petrochemical industry is in the production of 1,3-butadiene. Butene is used in the plastics industry to make both homopolymers and copolymers. Another use of 1-butene is in the production of solvents containing four carbons such as secondary butyl alcohol and methyl ethyl ketone (MEK).
Definition
ChEBI: A butene with unsaturation at position 1.
Production Methods
Most butenes are produced in the cracking process in refineries along with other C-4 fractions such as the butanes. Butenes are separated from other compounds and each other by several methods. Isobutene is separated from normal butanes by absorption in a sulfuric acid solution. Normal butenes can be separated from butanes by fractionation. The close boiling points of butanes and butenes make straight fractional distillation an inadequate separation method, but extractive distillation can be used. Butenes can also be prepared from the dehydrogenation (elimination of hydrogen) of butane.
General Description
Colorless gas.
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
The unsaturated aliphatic hydrocarbons, such as 1-BUTENE, are generally much more reactive than the alkanes. Strong oxidizers may react vigorously with them. Reducing agents can react exothermically to release gaseous hydrogen. In the presence of various catalysts (such as acids) or initiators, compounds in this class can undergo very exothermic addition polymerization reactions. May react with oxidizing materials. Aluminum borohydride reacts with alkenes and in the presence of oxygen, combustion is initiated even in the absence of moisture.
Health Hazard
Exposures to 1-butene cause the effects of an asphyxiant and/or an anesthetic (at high concentrations). Workers exposed to 1-butene develop eye irritation.
Fire Hazard
1-BUTENE is flammable. Vapor is heavier than air and may travel long distances to an ignition source and flash back.
Flammability and Explosibility
Extremely flammable
Source
In exhaust of gasoline-powered engines (1.8 vol % of total exhaust hydrocarbons)
(quoted, Verschueren, 1983). Detected in California Phase II reformulated gasoline at a
concentration of 170 mg/kg (Schauer et al., 2002).
Schauer et al. (2001) measured organic compound emission rates for volatile organic
compounds, gas-phase semi-volatile organic compounds, and particle-phase organic compounds
from the residential (fireplace) combustion of pine, oak, and eucalyptus. The gas-phase emission
rate of 1-butene was 90.7 mg/kg of pine burned. Emission rates of 1-butene were not measured
during the combustion of oak and eucalyptus.
Reported as an impurity (0.1 wt %) in 99.4 wt % trans-2-butene (Chevron Phillips, 2004).
Environmental Fate
Biological. Biooxidation of 1-butene may occur yielding 3-buten-1-ol, which may oxidize to
give 3-butenoic acid (Dugan, 1972). Washed cell suspensions of bacteria belonging to the genera
Mycobacterium, Nocardia, Xanthobacter, and Pseudomonas and growing on selected alkenes
metabolized 1-butene to 1,2-epoxybutane (Van Ginkel et al., 1987).
Photolytic. Products identified from the photoirradiation of 1-butene with nitrogen dioxide in air
are epoxybutane, 2-butanone, propanal, ethanol, ethyl nitrate, carbon monoxide, carbon dioxide,
methanol, and nitric acid (Takeuchi et al., 1983).
The following rate constants were reported for the reaction of 1-butene and OH radicals in the
atmosphere: 1.0 x 10-17 cm3/molecule?sec (Bufalini and Altshuller, 1965); 2.70 x 10-11
cm3/molecule?sec (Atkinson et al., 1979); 3.14 x 10-11 cm3/molecule?sec (Atkinson, 1990; Sablji?
and Güsten, 1990). Reported photooxidation reaction rate constants for the reaction of 1-butene
and ozone are 1.23 x 10-17, 1.0 x 10-17, 1.03 x 10-17 cm3/molecule?sec (Adeniji et al., 1981). Based
on the reaction of 1-butene and OH radicals gas phase, the atmospheric lifetime was estimated to
be 5.5 h in summer sunlight.
Chemical/Physical. Complete combustion in air yields carbon dioxide and water. Incomplete
combustion will generate carbon monoxide.
Hydrolysis in water is not expected to be signicant because 1-butene is very volatile.
Precautions
When working with 1-butene, occupational workers should wear proper protectives, preferably a NIOSH-approved full-face positive pressure supplied-air respirator or a self contained breathing apparatus (SCBA). Workers should not wear contact lenses.
Properties of 1-BUTENE
| Melting point: | −185 °C(lit.) |
| Boiling point: | −6.3 °C(lit.) |
| Density | 0.5951 |
| vapor density | 1.93 (vs air) |
| vapor pressure | 1939 mm Hg ( 21.1 °C) |
| refractive index | 1.3962 |
| Flash point: | 80 |
| storage temp. | -20°C Freezer |
| solubility | Soluble in alcohol, benzene, and ether (Weast, 1986) |
| form | gas |
| pka | >14 (Schwarzenbach et al., 1993) |
| color | Colorless to Almost colorless |
| Odor | Slightly aromatic |
| Odor Threshold | 0.36ppm |
| explosive limit | 9.3% |
| Water Solubility | 222 mg/kg at 25 °C (shake flask-GC, McAuliffe, 1966) |
| FreezingPoint | -185.35℃ |
| Merck | 14,1519 |
| BRN | 1098262 |
| Henry's Law Constant | (atm?m3/mol):
0.25 at 25 °C (Hine and Mookerjee, 1975) |
| Stability: | Volatile |
| CAS DataBase Reference | 106-98-9(CAS DataBase Reference) |
| EPA Substance Registry System | 1-Butene (106-98-9) |
Safety information for 1-BUTENE
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Gas Cylinder Compressed Gases GHS04 |
| GHS Hazard Statements |
H220:Flammable gases H280:Gases under pressure |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P410+P403:Protect from sunlight. Store in a well-ventilated place. |
Computed Descriptors for 1-BUTENE
| InChIKey | VXNZUUAINFGPBY-UHFFFAOYSA-N |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 106-98-9 1-Butene 98%View Details
106-98-9 1-Butene 98%View Details
106-98-9 -
 106-98-9 98%View Details
106-98-9 98%View Details
106-98-9 -
 1-Butene (ca. 10% in Hexane) CAS 106-98-9View Details
1-Butene (ca. 10% in Hexane) CAS 106-98-9View Details
106-98-9 -
 1-Butene (ca. 10% in Toluene) CAS 106-98-9View Details
1-Butene (ca. 10% in Toluene) CAS 106-98-9View Details
106-98-9 -
 1-Butene (ca. 8% in Tetrahydrofuran, ca. 1.3 mol/L) CAS 106-98-9View Details
1-Butene (ca. 8% in Tetrahydrofuran, ca. 1.3 mol/L) CAS 106-98-9View Details
106-98-9 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
