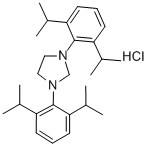
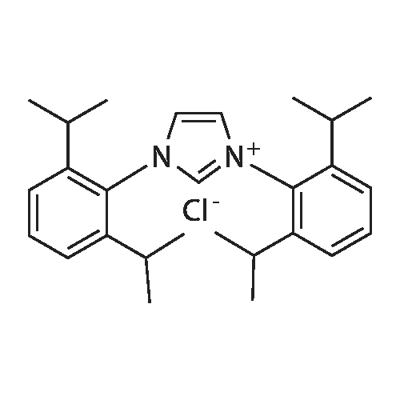
1,3-Bis(2,4,6-trimethylphenyl)imidazolium chloride
Synonym(s):1,3-Bis(mesityl)imidazolium chloride;1,3-Dihydro-1,3-dimesityl-2H-imidazol-2-ylidene monohydrochloride;1,3-Dimesitylimidazolium chloride
- CAS NO.:141556-45-8
- Empirical Formula: C21H25ClN2
- Molecular Weight: 340.89
- MDL number: MFCD02684541
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-10-08 13:17:58
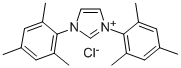
What is 1,3-Bis(2,4,6-trimethylphenyl)imidazolium chloride ?
Chemical properties
off-white to beige powder
The Uses of 1,3-Bis(2,4,6-trimethylphenyl)imidazolium chloride
1,3-Bis(2,4,6-trimethylphenyl)imidazolium chloride is used as a phosphine-free ligand in various metal-catalyzed coupling reactions, often with advantageous results in difficult cases. For use in the Pd-catalyzed cross-coupling of aryl Grignards with aryl chlorides (Kumada reaction). Many examples have been recorded of the use of NHC ligands in the Suzuki coupling reaction, for examples utilizing 1,3-dimesitylimidazol-2-ylidene, in the coupling of arylboronic acids with relatively unreactive aryl chlorides.
What are the applications of Application
1,3-Bis(2,4,6-trimethylphenyl)imidazolium chloride is a ligand for arylation of aldehydes
Preparation
In a flask, the imine(3 g, 10 mmol) was dissolved in tetrahydrofuran(25 ml), followed by dropwise addition of chloromethyl ethyl ether(1.04 g, 11 mmol). the mixture was stirred under N2 at 40 °C for 18 h, and then ethyl ether(25 ml) was added to separate white solid. The solid was filtered and washed with ethyl ether. Finally, the white solid was dried under vacuum affording 1,3-Bis(2,4,6-trimethylphenyl)imidazolium chloride.
Reactions
Precursor to the nucleophilic carbene that serves as a bulky, electron-rich "phosphine mimic" for metal-catalyzed reactions.
(a) Palladium-catalyzed Suzuki cross-coupling of aryl chlorides.
(b) Palladium-catalyzed Kumada cross-coupling of aryl chlorides.
(c) Ruthenium-carbene catalysts for ring-closing metathesis.
(d) Suzuki coupling of aryltrimethylammonium salts.
(e) Sonogashira coupling of aryl bromides.
Precursor to a nucleophilic carbene that serves as catalyst.
Ligand for arylation of aldehydes.
Ligand for carbene catalyzed intermolecular arylation of C-H bonds.
Catalyst for boron conjugate additions to cyclic and acyclic α,β-unsaturated carbonyls.
Ligand for dehydrogenative cyclocondensation of aldehydes, alkynes, and dialkylsilanes.
Precursor for carbene for conjugate silylation of alpha, beta-unsaturated carbonyls.


Properties of 1,3-Bis(2,4,6-trimethylphenyl)imidazolium chloride
| Melting point: | >300 °C (lit.) |
| Boiling point: | 499.2°C (rough estimate) |
| Density | 1.0279 (rough estimate) |
| refractive index | 1.5940 (estimate) |
| storage temp. | under inert gas (nitrogen or Argon) at 2–8 °C |
| form | Powder |
| color | Off-white to beige |
| Water Solubility | Slightly soluble in water. |
| CAS DataBase Reference | 141556-45-8(CAS DataBase Reference) |
Safety information for 1,3-Bis(2,4,6-trimethylphenyl)imidazolium chloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 1,3-Bis(2,4,6-trimethylphenyl)imidazolium chloride
| InChIKey | OTOSIXGMLYKKOW-UHFFFAOYSA-M |
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 1,3-Dimesitylimidazolium Chloride CAS 141556-45-8View Details
1,3-Dimesitylimidazolium Chloride CAS 141556-45-8View Details
141556-45-8 -
 1,3-Bis(2,4,6-trimethylphenyl)imidazolium chloride CAS 141556-45-8View Details
1,3-Bis(2,4,6-trimethylphenyl)imidazolium chloride CAS 141556-45-8View Details
141556-45-8 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1