1,2,3,4-Tetrahydronaphthalene
Synonym(s):1,2,3,4-Tetrahydronaphthalene;1,2,3,4-Tetrahydronaphthalene solution;Tetralin;Tetralin in dimethyl sulfoxide;Tetralin solvent
- CAS NO.:119-64-2
- Empirical Formula: C10H12
- Molecular Weight: 132.2
- MDL number: MFCD00001733
- EINECS: 204-340-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-06-13 14:48:16
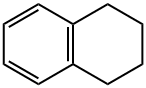
What is 1,2,3,4-Tetrahydronaphthalene?
Chemical properties
colourless liquid with a mouldy smell
Chemical properties
Tetralin or 1,2,3,4-tetrahydronaphthalene is a flammable liquid.
The Uses of 1,2,3,4-Tetrahydronaphthalene
1,2,3,4-Tetrahydronaphthalene, is used as an intermediate for organic synthesis, solvent. It is used as a solvent.It is also used for the laboratory synthesis of dry HBr gas.
The Uses of 1,2,3,4-Tetrahydronaphthalene
Degreasing agent. Solvent for naphthalene, fats, resins, oils, waxes, used instead of turpentine in lacquers, shoe polishes, floor waxes.
The Uses of 1,2,3,4-Tetrahydronaphthalene
As a solvent for fats and oils and as an alternative to turpentine in polishes and paint; insecticide.
What are the applications of Application
1,2,3,4-Tetrahydronaphthalene is A chemical produced by the catalytic hydrogenation of purified naphthalene.
Definition
ChEBI: An ortho-fused bicyclic hydrocarbon that is 1,2,3,4-tetrahydro derivative of naphthalene.
Production Methods
Tetralin is prepared by the catalytic hydrogenation of naphthalene or during acidic, catalytic hydrocracking of phenanthrene. At 700℃, tetralin yields tars that contain appreciable quantities of 3,4-benzopyrene (172a).
Synthesis Reference(s)
Journal of the American Chemical Society, 111, p. 314, 1989 DOI: 10.1021/ja00183a048
Tetrahedron Letters, 12, p. 1853, 1971
General Description
A light colored liquid. May be irritating to skin, eyes and mucous membranes. Flash point 100-141°F.
Air & Water Reactions
Flammable.
Reactivity Profile
1,2,3,4-Tetrahydronaphthalene may react vigorously with strong oxidizing agents. May react exothermically with reducing agents to release hydrogen gas. Oxidizes readily in air to form unstable peroxides that may explode spontaneously [Bretherick 1979 p.151-154].
Hazard
Irritant to eyes and skin; narcotic in high concentration.
Health Hazard
Liquid may cause nervous disturbance, green coloration of urine, and skin and eye irritation
Carcinogenicity
In male and female F344/N and NBR rats exposed to tetralin at concentrations of 0, 30, 60, or 120 ppm, 6 h plus T90 (12 min) per day, 5 days per week for 105 weeks, there were slightly increased incidences of cortical renal tubule adenoma in male rats. The incidence of cortical renal tubule adenomawas also significantly increased in the 120 ppm group. Exposure of male and female B6C3F1 mice to tetralin at concentrations of 0, 30, 60, or 120 ppm, 6 h plus T90 (12 min) per day, 5 days per week for 105 weeks and additional groups of male and female mice to the same concentrations for 12 months led to increased incidence of hemangiosarcoma of the spleen in 120 ppm females (172b).
Purification Methods
Wash tetralin with successive portions of conc H2SO4 until the acid layer is no longer coloured, then wash it with aqueous 10% Na2CO3, and then distilled water. Dry (CaSO4 or Na2SO4), filter, reflux and fractionally distil it under under reduced pressure from sodium or BaO. It can also be purified by repeated fractional freezing. Bass [J Chem Soc 3498 1964] freed tetralin, purified as above, from naphthalene and other impurities by conversion to ammonium tetralin-6-sulfonate. Concentrated H2SO4 (150mL) is added slowly to stirred tetralin (272mL) which is then heated on a water bath for about 2hours for complete solution. The warm mixture, when poured into aqueous NH4Cl solution (120g in 400mL water), gives a white precipitate which, after filtering off, is crystallised from boiling water, washed with 50% aqueous EtOH and dried at 100o. Evaporation of its boiling aqueous solution on a steam bath removes traces of naphthalene. The pure salt (229g) is mixed with conc H2SO4 (266mL) and steam distilled from an oil bath at 165-170o. An ether extract of the distillate is washed with aqueous Na2SO4, and the ether is evaporated, prior to distilling the tetralin from sodium. Tetralin has also been purified via barium tetralin-6-sulfonate, conversion to the sodium salt and decomposed in 60% H2SO4 using superheated steam. [Beilstein 5 H 491, 5 III 1219, 5 IV 1388.]
Properties of 1,2,3,4-Tetrahydronaphthalene
| Melting point: | -35 °C (lit.) |
| Boiling point: | 207 °C (lit.) |
| Density | 0.973 g/mL at 25 °C (lit.) |
| vapor density | 4.55 (vs air) |
| vapor pressure | 0.18 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 171 °F |
| storage temp. | Store below +30°C. |
| solubility | 0.045g/l |
| form | Fluid |
| color | Colorless |
| Odor | pungent menthol odor |
| explosive limit | 0.8%, 100°F |
| Odor Threshold | 0.0093ppm |
| Water Solubility | INSOLUBLE |
| Sensitive | Air Sensitive |
| Merck | 14,9221 |
| BRN | 1446407 |
| Dielectric constant | 2.77 |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 119-64-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Tetralin(119-64-2) |
| EPA Substance Registry System | 1,2,3,4-Tetrahydronaphthalene (119-64-2) |
Safety information for 1,2,3,4-Tetrahydronaphthalene
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H304:Aspiration hazard H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H351:Carcinogenicity H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P273:Avoid release to the environment. P331:Do NOT induce vomiting. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 1,2,3,4-Tetrahydronaphthalene
1,2,3,4-Tetrahydronaphthalene manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Tetrahydronaphthalene CAS 119-64-2View Details
Tetrahydronaphthalene CAS 119-64-2View Details
119-64-2 -
 Tetraline, puriss+ CAS 119-64-2View Details
Tetraline, puriss+ CAS 119-64-2View Details
119-64-2 -
 Tetraline CAS 119-64-2View Details
Tetraline CAS 119-64-2View Details
119-64-2 -
![1,2,3,4-Tetrahydronaphthalene [for Spectrophotometry] CAS 119-64-2](https://img.chemicalbook.in//Content/image/CP5.jpg) 1,2,3,4-Tetrahydronaphthalene [for Spectrophotometry] CAS 119-64-2View Details
1,2,3,4-Tetrahydronaphthalene [for Spectrophotometry] CAS 119-64-2View Details
119-64-2 -
 TETRALIN For Synthesis CAS 119-64-2View Details
TETRALIN For Synthesis CAS 119-64-2View Details
119-64-2 -
 1,2,3,4-Tetrahydronaphthalene CAS 119-64-2View Details
1,2,3,4-Tetrahydronaphthalene CAS 119-64-2View Details
119-64-2 -
 1,2,3,4-Tetrahydronaphthalene CAS 119-64-2View Details
1,2,3,4-Tetrahydronaphthalene CAS 119-64-2View Details
119-64-2 -
 1,2,3,4-Tetrahydronaphthalene CAS 119-64-2View Details
1,2,3,4-Tetrahydronaphthalene CAS 119-64-2View Details
119-64-2
