Borane-tetrahydrofuran complex
- CAS NO.:14044-65-6
- Empirical Formula: C4H11BO
- Molecular Weight: 85.94
- MDL number: MFCD00012429
- EINECS: 237-881-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
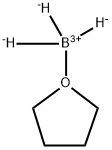
What is Borane-tetrahydrofuran complex?
Description
Borane tetrahydrofuran complex (BH3-THF) is widely used as a reducing agent in organic synthesis. It is also used as a reagent in hydroboration reactions.It is a charge-transfer complex that is a useful surrogate for diborane1 in organic synthesis. It can be used to reduce carboxylic acids to alcohols or nitriles to primary amines. It reacts with olefins to add the BH2 functional group. Alkyl- or arylboranes formed in this way can further react with unsaturated compounds such as olefins, imines, ketones, and alkynes (the hydroboration reaction) to make useful boron-containing intermediates.
Description
Borane–tetrahydrofuran (BH3–THF) is a charge-transfer complex that is a useful surrogate for diborane1 in organic synthesis. It can be used to reduce carboxylic acids to alcohols or nitriles to primary amines. It reacts with olefins to add the BH2 functional group. Alkyl- or arylboranes formed in this way can further react with unsaturated compounds such as olefins, imines, ketones, and alkynes (the hydroboration reaction) to make useful boron-containing intermediates.
Charge-transfer complexes such as BH3–THF form when a Lewis base (THF in this case) “donates” its unshared electron pair to an electron-deficient Lewis acid such as borane. The electrostatic attraction between the components is not as strong as a covalent bond; but in many cases it is sufficient to make the complex behave as a covalent compound. As an example, BH3–THF can be distilled without decomposing.
BH3–THF’s relative stability makes it a convenient way to use extremely toxic and flammable diborane. But it is not without its own risks. In particular, it reacts violently with water to produce copious amounts of hydrogen. As a result, BH3–THF is normally sold as a 1 M (≈10 wt%) solution in THF. It is available from numerous chemical supply houses worldwide.
1. Under standard conditions, borane dimerizes to produce the more electronically stable diborane.
Description
Borane-tetrahydrofuran (BH3-THF) is a complex of borane with tetrahydrofuran, and is generally purchased as a solution in THF. BH3-THF can decompose violently, therefore BH3-THF is typically only available in 1 M concentration. It is recommended that BH3-THF solutions be stored at 0-5 C, and that reactions are performed at under 35 C. Borane-dimethylsulfide (BH3-SMe2) is more stable than BH3-THF and is available in much higher concentrations (10 M).
Chemical properties
colourless liquid
The Uses of Borane-tetrahydrofuran complex
Borane-tetrahydrofuran complex is used to reduce Nylon surface amide groups to secondary amines.It is an important reagent used in the reduction of certain functional groups viz. aldehyde, ketone, carboxylic acid, amide, oxime, imine and nitrile. It is also used as hydro borating agent. It acts as a borane source for oxazaborolidine catalyzed asymmetric reductions.
The Uses of Borane-tetrahydrofuran complex

To a 0.24M THF suspension of the SM (71.7 mmol) at 0 C was added BH3-THF (1M, 220 mmol). The reaction mixture was stirred under Ar at RT for 66 h then quenched by addition of EtOH (15 mL) at 0 C and stirred an additional 15 min. The mixture was poured into H2O and extracted with DCM. The combined organics were washed with brine, dried (Na2SO4), and concentrated to provide the crude product as a white solid. [10.16 g, 62%]
The Uses of Borane-tetrahydrofuran complex
Borane-tetrahydrofuran complex is an important reagent used in the reduction of certain functional groups viz. aldehyde, ketone, carboxylic acid, amide, oxime, imine and nitrile. It is also used as hydro borating agent. It acts as a borane source for oxazaborolidine catalyzed asymmetric reductions. It is utilized to reduce nylon surface amide groups to secondary amines.
What are the applications of Application
New, Safer, NIMBA-Stabilized BH3 THF Solutions
BH3-THF can be used as a reducing agent for the reduction of various functional groups such as carboxylic acids, aldehydes, ketones, esters, acid chlorides, nitriles, epoxides, amides, lactones, oximes, and imines into corresponding alcohols and amines. Grignard reagents, arylmercury, arylthalium, and allyl and propargyllithium compounds react with BH3?THF to give organoboranes, which can be oxidized to the corresponding alcohols, phenols, and 1,3-diols.
It can also be used:
To synthesize the chiral borane catalyst, which is used in the enantioselective halo-aldol reaction.
To prepare 9-unsubstituted acridines by reduction of corresponding acridones.
To reduce nylon surface amide groups to secondary amines.
Reactions
Borane-tetrahydrofuran complex (BTHF) is a valuable reagent for the reduction of functional groups and for hydroboration reactions with carbon-carbon double and triple bonds. Functional groups that are readily reduced by BTHF include aldehyde, ketone, carboxylic acid, amide, oxime, imine, and nitrile. The carboxylic acid group is reduced at a faster rate than most groups including non-conjugated alkene. Conjugated α,β-unsaturated carboxylic acids give saturated alcohols as the major products.
Ketones and the carbonyl of enones are effectively reduced with borane-tetrahydrofuran. The addition of borohydride to the reaction solution is advantageous for accelerated reduction as well as higher selectivity towards carbonyl reduction in conjugated and non-conjugated enones.
Asymmetric ketone reduction using chiral oxazaborolidine catalysts was recently reviewed. Work at Callery with BTHF improved on reaction conditions to provide consistent results in the reduction.
General Description
Borane tetrahydrofuran complex (BH3-THF) is widely used as a reducing agent in organic synthesis. It is also used as a reagent in hydroboration reactions.
Precautions
Air and moisture sensitive. Forms explosive peroxides in contact with air. Incompatible with acids, acid chlorides, acid anhydrides, oxidizing agents and alcohols. On hydrolysis, it forms hydrogen and boric acid.
Properties of Borane-tetrahydrofuran complex
| Melting point: | -17 °C |
| Boiling point: | 35 °C |
| Density | 0.898 g/mL at 25 °C |
| Flash point: | 1 °F |
| storage temp. | Store at <= 20°C. |
| solubility | reacts violently |
| appearance | colorless liquid |
| form | Liquid |
| color | Colorless |
| Water Solubility | REACTS |
| Sensitive | Air & Moisture Sensitive |
| BRN | 3668402 |
| Exposure limits | ACGIH: TWA 50 ppm; STEL 100 ppm (Skin) OSHA: TWA 200 ppm(590 mg/m3) NIOSH: IDLH 2000 ppm; TWA 200 ppm(590 mg/m3); STEL 250 ppm(735 mg/m3) |
| Stability: | Stable, but may form explosive peroxides in contact with air. Incompatible with acids, acid chlorides, acid anhydrides, oxidizing agents, alcohols. Reacts violently with water. Highly flammable. Store under inert gas. |
| CAS DataBase Reference | 14044-65-6(CAS DataBase Reference) |
| EPA Substance Registry System | Boron, trihydro(tetrahydrofuran)-, (T-4)- (14044-65-6) |
Safety information for Borane-tetrahydrofuran complex
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H260:Substances And Mixtures Which, In Contact With Water,Emit Flammable Gases H302:Acute toxicity,oral H315:Skin corrosion/irritation H318:Serious eye damage/eye irritation H319:Serious eye damage/eye irritation H333:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H351:Carcinogenicity |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P223:Keep away from any possible contact with water, because of violent reaction and possible flash fire. P280:Wear protective gloves/protective clothing/eye protection/face protection. P231+P232:Handle under inert gas. Protect from moisture. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P370+P378:In case of fire: Use … for extinction. P405:Store locked up. P402+P404:Store in a dry place. Store in a closed container. P403+P235:Store in a well-ventilated place. Keep cool. |
Computed Descriptors for Borane-tetrahydrofuran complex
Borane-tetrahydrofuran complex manufacturer
Sanpra Synthesis Private Limited
New Products
1-Amino-1-cyclohexanecarboxylic acid 2-Hydroxyacetamide Ethyl 5-cyclopropyl-1H-pyrazole-3-carboxylate 2,4,6-Trichloroaniline ELECTROLYTIC IRON POWDER 1-Aminocyclobutanecarboxylic acid 1-(2-Ethoxyethyl)-2-(piperidin-4-yl)-1H-benzo[d]imidazole hydrochloride Decanonitrile tert-butyl 4-(1H-benzo[d]iMidazol-2-yl)piperidine-1-carboxylate N,N'-diallyl-1,3-diaminopropanedihydrochloride 3-Iodo-6-nitroindazole (4-(4-Fluorophenoxy)phenyl)methanamine Tert-butyl N-(pent-4-yn-2-yl) carbamate 2-Chloro-3-nitropyridine 5-Bromo-2,3-dimethoxypyridine 2-chloro-4-methyl-5-nitro pyridine 2,5-dibromopyridine terephthalonitrile 5-Bromo-4-chloro-2(1h)-pyridinone, 5-Fluoro-2-Oxindole N, N-Carbonyldiimidazole (CDI) 5-Cyanophthalide 10-Methoxy-5H-dibenz[b,f]azepine 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA]Related products of tetrahydrofuran



You may like
-
 14044-65-6 Borane THF complex 1.0 molar-2.0 molar in THF 98%View Details
14044-65-6 Borane THF complex 1.0 molar-2.0 molar in THF 98%View Details
14044-65-6 -
 14044-65-6 98%View Details
14044-65-6 98%View Details
14044-65-6 -
 Borane THF 1.0M in THF CAS 14044-65-6View Details
Borane THF 1.0M in THF CAS 14044-65-6View Details
14044-65-6 -
 Borane CAS 14044-65-6View Details
Borane CAS 14044-65-6View Details
14044-65-6 -
 Borane-tetrahydrofuran complex 98%View Details
Borane-tetrahydrofuran complex 98%View Details
14044-65-6 -
 Borane - Tetrahydrofuran Complex (8.5% in Tetrahydrofuran, ca. 0.9mol/L) (stabilized with Sodium Borohydride) CAS 14044-65-6View Details
Borane - Tetrahydrofuran Complex (8.5% in Tetrahydrofuran, ca. 0.9mol/L) (stabilized with Sodium Borohydride) CAS 14044-65-6View Details
14044-65-6 -
 Borane CAS 14044-65-6View Details
Borane CAS 14044-65-6View Details
14044-65-6 -
 Borane tetrahydrofuran complex solution CAS 14044-65-6View Details
Borane tetrahydrofuran complex solution CAS 14044-65-6View Details
14044-65-6