1,2,3-Trichloropropane
Synonym(s):Glycerol trichlorohydrin;Trichlorohydrin
- CAS NO.:96-18-4
- Empirical Formula: C3H5Cl3
- Molecular Weight: 147.43
- MDL number: MFCD00000946
- EINECS: 202-486-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
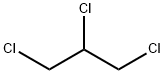
What is 1,2,3-Trichloropropane?
Description
1,2,3-Trichloropropane is a synthetic chemical that is also known as allyl trichloride, glycerol trichlorohydrin, and trichlorohydrin. It is a colourless, heavy liquid with a sweet but strong chloroform-like odour and is combustible. It is slightly soluble in water but soluble in chloroform, diethyl ether, and ethanol. On contact with heat/ fire, It releases off irritating or toxic fumes (or gases). It evaporates very quickly and small amounts dissolve in water. It is mainly used to make other chemicals. 1,2,3-Trichloropropane was used in the past mainly as a solvent and extractive agent, including as a paint and varnish remover and as a cleaning and degreasing agent. It is now used mainly as a chemical intermediate, for example, in the production of polysulphone liquid polymers, dichloropropene and hexafluoropropylene and as a cross-linking agent in the synthesis of polysulphides.
Chemical properties
1,2,3-Trichloropropane is a colorless liquid with a strong acid odor. slightly soluble in water; dissolves oils, fats, waxes, chlorinated rubber, and numerous resins. autoign temp 580F (304°C). Combustible.
Physical properties
Clear, colorless liquid with a strong, chloroform-like odor
The Uses of 1,2,3-Trichloropropane
1,2,3-Trichloropropane is used as a solventand as an intermediate in organic synthesis.
The Uses of 1,2,3-Trichloropropane
Paint and varnish remover, solvent, degreasing agent.
The Uses of 1,2,3-Trichloropropane
Intermediate in the manufacture of pesticides and polysulfide rubbers; formerly used as a solvent and extractive agent.
Definition
ChEBI: 1,2,3-Trichloropropane is an organochlorine compound.
General Description
Colorless liquid with a strong acid odor. Denser than water and slightly soluble in water. Hence sinks in water.
Reactivity Profile
1,2,3-Trichloropropane is sensitive to prolonged exposure to light. Sensitive to heat. May react with active metals, strong caustics and oxidizing agents. Attacks some plastics, rubber and some coatings .
Health Hazard
Inhalation of vapor causes anesthesia, dizziness, and nausea. Vapor is highly irritating by inhalation routes and moderately irritating by dermal routes. Exposure of eyes to vapor may result in slight, transient injury to the cornea.
Health Hazard
Inhalation of its vapors can produce depres sion of the central nervous system, which canprogress to narcosis and convulsion as theconcentration increases. A 30-minute expo sure to a 5000-ppm concentration causedconvulsions in rats. Acute as well as chronicexposure to high concentrations can causeliver damage. 1,2,3-Trichloropropane is moretoxic than its 1,1,1-isomer. Acute oral tox icity is moderate, with LD50 values rang ing between 300 and 550 mg/kg in differentspecies of experimental animals. The liquidis a strong irritant to the eyes.
Fire Hazard
Combustible liquid; flash point (closed cup) 73°C (164°F), (open cup) 82°C (180°F). Vapors of 1,2,3-trichloropropane form explo sive mixtures with air, with LEL and UEL values of 3.2% and 12.6% by volume in air, respectively. The compound reacts vigorously with alkali metals, powdered magnesium, or aluminum; caustic alkalies; and oxidizers.
Flammability and Explosibility
Non flammable
Safety Profile
Confirmed carcinogen. Poison by ingestion. Moderately toxic by inhalation and skin contact. Experimental reproductive effects. A skin and severe eye irritant. Mutation data reported. Moderately flammable by heat, flames (sparks), or powerful oxidizers. See also ALLYL COMPOUNDS and CHLORINATED HYDROCARBONS, ALIPHATIC. When heated to decomposition it yields hghly toxic Cl-. To fight fre, use water (as a blanket), spray, mist, dry chemical.
Potential Exposure
Trichloropropane dissolves oils, fats, waxes, chlorinated rubber; and numerous resins; it is used as a paint and varnish remover; a solvent; and a degreasing agent.
Carcinogenicity
1,2,3-Trichloropropane is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.
Environmental Fate
Chemical/Physical. The hydrolysis rate constant for 1,2,3-trichloropropane at pH 7 and 25 °C
was determined to be 1.8 x 10-6/h, resulting in a half-life of 43.9 yr (Ellington et al., 1988). The
hydrolysis half-lives decrease at varying pHs and temperature. At 87 °C, the hydrolysis half-lives
at pH values of 3.07, 7.12, and 9.71 were 21.1, 11.6, and 0.03 d, respectively (Ellington et al.,
1986). By analogy to 1,2-dibromo-2-chloropropane, the following hydrolysis products would be
formed: 2,3-dichloro-1-propanol, 2,3-dichloropropene, epichlorohydrin, 1-chloro-2,3-
dihydroxypropane, glycerol, 1-hydroxy-2,3-propylene oxide, 2-chloro-3-hydroxypropene, and
HCl (Kollig, 1993).
The volatilization half-life of 1,2,3-trichloropropane (1 mg/L) from water at 25 °C using a
shallow-pitch propeller stirrer at 200 rpm at an average depth of 6.5 cm was 56.1 min (Dilling,
1977).
Shipping
UN2810 Toxic liquids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Toxicity evaluation
It is currently believed that TCP itself has very low activity, while its metabolites mainly cause toxicity, carcinogenicity, and other effects. The liver can metabolize TCP by cytochrome P450 enzymes to give reactive intermediates, which bind to glutathione or other protein for excretion. The reactive intermediates can bind to DNA, proteins, and other molecules and cause hepatocellular damage, gene mutation, and organ dysfunction. Activation of the molecule may occur by reaction with glutathione. The metabolites (e.g., dichloroacetone) accumulated through glutathione depletion and consequently covalent binding to DNA and other macromolecules such as microsomal proteins.
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Violent decomposition with chemically active metals; strong bases. Keep away from chlorinated rubber, resins and waxes; and sunlight.
Waste Disposal
Incineration, preferably after mixing with another combustible fuel. Care must be exercised to assure complete combustion to prevent the formation of phosgene. An acid scrubber is necessary to remove the halo acids produced.
Properties of 1,2,3-Trichloropropane
| Melting point: | -14 °C (lit.) |
| Boiling point: | 156 °C (lit.) |
| Density | 1.387 g/mL at 25 °C (lit.) |
| vapor pressure | 3.1 at 25 °C (Banerjee et al., 1990) |
| refractive index | n |
| Flash point: | 180 °F |
| storage temp. | 2-8°C |
| solubility | Soluble in alcohol and ether (Weast, 1986); miscible with propyl chloride, carbon tetrachloride,
and chloroform. |
| form | neat |
| color | Colorless to Almost colorless |
| Water Solubility | 2 g/L (25 ºC) |
| BRN | 1732068 |
| Henry's Law Constant | 34.5 at 25.0 °C (mole fraction ratio-GC, Leighton and Calo, 1981) |
| Exposure limits | TLV-TWA 10 ppm (~60 mg/m3) (ACGIH),
50 ppm (MSHA, OSHA, and NIOSH); IDLH
1000 ppm (NIOSH). |
| Dielectric constant | 2.3(29℃) |
| CAS DataBase Reference | 96-18-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Propane, 1,2,3-trichloro-(96-18-4) |
| IARC | 2A (Vol. 63) 1995 |
| EPA Substance Registry System | 1,2,3-Trichloropropane (96-18-4) |
Safety information for 1,2,3-Trichloropropane
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation H341:Germ cell mutagenicity H350:Carcinogenicity H372:Specific target organ toxicity, repeated exposure H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 1,2,3-Trichloropropane
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran





You may like
-
 1,2,3-Trichloropropane CAS 96-18-4View Details
1,2,3-Trichloropropane CAS 96-18-4View Details
96-18-4 -
 1,2,3-Trichloropropane CAS 96-18-4View Details
1,2,3-Trichloropropane CAS 96-18-4View Details
96-18-4 -
 1,2,3-Trichloropropane CAS 96-18-4View Details
1,2,3-Trichloropropane CAS 96-18-4View Details
96-18-4 -
 1,2,3-Trichloropropane solution CAS 96-18-4View Details
1,2,3-Trichloropropane solution CAS 96-18-4View Details
96-18-4 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1