1,2-NAPHTHOQUINONE
Synonym(s):β-Naphthoquinone
- CAS NO.:524-42-5
- Empirical Formula: C10H6O2
- Molecular Weight: 158.15
- MDL number: MFCD00001698
- EINECS: 208-360-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-04 15:17:56
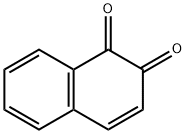
What is 1,2-NAPHTHOQUINONE?
Chemical properties
brown powder
The Uses of 1,2-NAPHTHOQUINONE
1,2-Naphthoquinone was employed as mediator during electrochemical mapping of redox activity in normal human breast (MCF-10A) cells by scanning electrochemical microscopy (SECM).
The Uses of 1,2-NAPHTHOQUINONE
Chemical reagent and intermediate.
The Uses of 1,2-NAPHTHOQUINONE
1,2-Naphthquinone is a highly reactive quinone species which aids in modulating cellular homeostasis and electrophilic signal transduction pathways.
What are the applications of Application
1,2-Naphthoquinone is a useful naphthalene for proteomics research
Definition
ChEBI: 1,2-naphthoquinone is the parent structure of the family of 1,2-naphthoquinones, in which the oxo groups of the quinone moiety are at positions 1 and 2 of the naphthalene ring. It is a metabolite of naphthalene and is found in diesel exhaust particles. It has a role as a carcinogenic agent and an aryl hydrocarbon receptor agonist. It derives from a hydride of a naphthalene.
Synthesis Reference(s)
Tetrahedron Letters, 25, p. 603, 1984 DOI: 10.1016/S0040-4039(00)99949-0
The Journal of Organic Chemistry, 51, p. 5390, 1986 DOI: 10.1021/jo00376a061
General Description
Golden yellow needles or brown powder. Decomposes to a bluish-black color on standing.
Air & Water Reactions
The neat chemical may be sensitive to prolonged exposure to air and light. Insoluble in water.
Reactivity Profile
Ketones, such as 1,2-NAPHTHOQUINONE, are reactive with many acids and bases liberating heat and flammable gases (e.g., H2). The amount of heat may be sufficient to start a fire in the unreacted portion of the ketone. Ketones react with reducing agents such as hydrides, alkali metals, and nitrides to produce flammable gas (H2) and heat. Ketones are incompatible with isocyanates, aldehydes, cyanides, peroxides, and anhydrides. They react violently with aldehydes, HNO3, HNO3 + H2O2, and HClO4.
Hazard
Irritant
Health Hazard
ACUTE/CHRONIC HAZARDS: 1,2-NAPHTHOQUINONE is an irritant. When heated to decomposition it emits acrid smoke and fumes.
Fire Hazard
Flash point data for 1,2-NAPHTHOQUINONE are not available. 1,2-NAPHTHOQUINONE is probably combustible.
Purification Methods
Crystallise the quinone from ether (red needles) or *benzene (orange leaflets). [Beilstein 7 IV 2417.]
Properties of 1,2-NAPHTHOQUINONE
| Melting point: | 139-142 °C (dec.) (lit.) |
| Boiling point: | 243.22°C (rough estimate) |
| Density | 1.450 g/cm3 (25℃) |
| refractive index | 1.5300 (estimate) |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), DMSO (Slightly) |
| form | Crystalline Mass or Waxy Solid |
| color | Colorless or white to pale yellow |
| Merck | 14,6394 |
| BRN | 606546 |
| Stability: | Light Sensitive |
| CAS DataBase Reference | 524-42-5(CAS DataBase Reference) |
| EPA Substance Registry System | 1,2-Naphthoquinone (524-42-5) |
Safety information for 1,2-NAPHTHOQUINONE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 1,2-NAPHTHOQUINONE
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 1,2-Naphthoquinone CAS 524-42-5View Details
1,2-Naphthoquinone CAS 524-42-5View Details
524-42-5 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Guanine , 99%View Details
Guanine , 99%View Details
73-40-5 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
