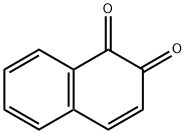
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | 1,2-naphthoquinone appears as golden yellow needles or brown powder. Decomposes to a bluish-black color on standing. (NTP, 1992) |
|---|---|
| Color/Form | Golden yellow needles |
| Odor | Odorless |
| Melting Point | 293 to 297 °F (Decomposes) (NTP, 1992) |
| Solubility | less than 1 mg/mL at 70 °F (NTP, 1992) |
| Density | 1.45 (NTP, 1992) - Denser than water; will sink |
| Vapor Pressure | 0.000099 [mmHg] |
| Decomposition | When heated to decomp it emits acrid smoke and fumes. |
| Kovats Retention Index | 264.1 |
| Other Experimental Properties | 1,2-Naphthoquinone is involatile /(non-volatile)/ in steam, and is a relatively weak oxidizing agent. |
| Chemical Classes | Other Classes -> Aromatic Ketones |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 158.15 g/mol |
|---|---|
| XLogP3 | 1.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 158.036779430 g/mol |
| Monoisotopic Mass | 158.036779430 g/mol |
| Topological Polar Surface Area | 34.1 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 253 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
1,2-naphthoquinone appears as golden yellow needles or brown powder. Decomposes to a bluish-black color on standing. (NTP, 1992)