1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine
Synonym(s):DOPE;Dioleoyl phosphatidylethanolamine;PE(18:1(9Z)/18:1(9Z));(9Z)-9-Octadecenoic acid (1R)-1-[[[(2-aminoethoxy)hydroxyphosphinyl]oxy]methyl]-1,2-ethanediyl ester;L -β,γ-Dioleoyl-α-cephalin
- CAS NO.:4004-05-1
- Empirical Formula: C41H78NO8P
- Molecular Weight: 744.05
- MDL number: MFCD16621009
- EINECS: 200-663-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-20 11:41:24
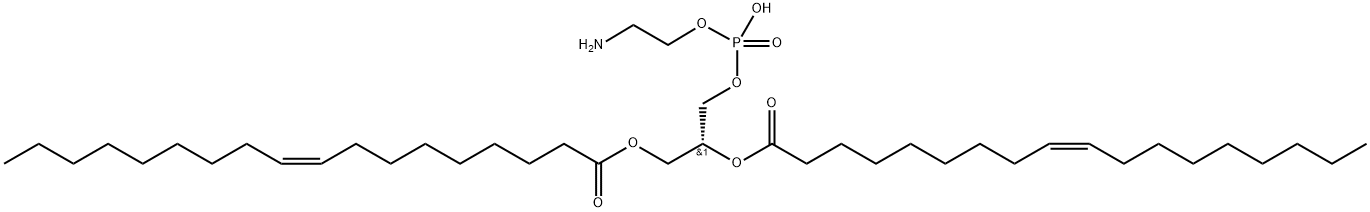
What is 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine?
Description
1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) is a neutral phospholipid and displays sensitivity towards pH change and has been used in the preparation of liposome and giant unilamellar vesicles (GUVs).
Phosphatidylethanolamine (PE) is formed primarily in the reaction of CDP-ethanolamine and diacylglycerol. PE is one of the major components of cell membranes in bacteria and in nervous system.
Chemical properties
Yellowish wax
The Uses of 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine
1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) forms heterogenous liposomes with N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium methylsulfate (DOTAP), which are used as delivery vehicles for therapeutic agents.
The Uses of 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine
1,2-Dioleoyl-sn-glycero-3-phosphatidylethanolamine (DOPE) is a synthetic analog of naturally-occurring PE containing 18:1 fatty acids at the sn-1 and sn-2 positions. DOPE can be used as an emulsifier to facilitate DNA-liposome complex transport across membranes. It is used in combination with cationic phospholipids to increase efficiency during DNA transfection studies as a non-viral method of gene delivery.
The Uses of 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine
18:1 (Δ9-Cis) PE (DOPE) has been used in the preparation of liposome for human uncoupling protein 1 (UCP1) reconstitution and for Caenorhabditis elegans autophagy related cysteine protease protein conjugation studies. It has also been used for the generation of giant unilamellar vesicles (GUVs) for septin induced deformation studies.
What are the applications of Application
1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine is a membrane phospholipid emulsifier to improve transfection of DNA
Definition
ChEBI: Dioleoyl phosphatidylethanolamine is a phosphatidylethanolamine in which the phosphatidyl acyl groups are both oleoyl.
General Description
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (18:1 (Δ9-Cis) PE (DOPE)) is a neutral phospholipid and displays sensitivity towards pH change. At acidic it is an inverted hexagonal micelle and transforms to spherical micelle at alkaline pH.
Biological Activity
DOPE in mixed membranes have been found to decrease fluorescence lifetime and increase acyl-chain order. DOPE is an essential component of cationic liposomes designed to deliver DNA into gliosarcoma and kidney cell lines (gene therapy).
Biochem/physiol Actions
DOPE in mixed membranes have been found to decrease fluorescence lifetime and increase acyl-chain order. DOPE is an essential component of cationic liposomes designed to deliver DNA into gliosarcoma and kidney cell lines (gene therapy).
storage
Store at -20°C
Properties of 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine
| Melting point: | 200°C(lit.) |
| Boiling point: | 759.2±70.0 °C(Predicted) |
| Density | 1.008±0.06 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | DMSO: 31.25 mg/mL (42.00 mM) |
| form | lyophilized powder |
| pka | 1.17±0.50(Predicted) |
| color | White to Light yellow |
| BRN | 1718431 |
Safety information for 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H331:Acute toxicity,inhalation H336:Specific target organ toxicity,single exposure; Narcotic effects H351:Carcinogenicity H372:Specific target organ toxicity, repeated exposure H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P273:Avoid release to the environment. P302+P352:IF ON SKIN: wash with plenty of soap and water. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine
| InChIKey | MWRBNPKJOOWZPW-XFBMKQTANA-N |
| SMILES | [C@H](COP(O)(=O)OCCN)(COC(=O)CCCCCCC/C=C\CCCCCCCC)OC(=O)CCCCCCC/C=C\CCCCCCCC |&1:0,r| |
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![PHOSPHATIDYLCHOLINE, L-ALPHA-DIOLEOYL, [DIOLEOYL-1-14C]](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB2727350.gif)

![L-3-PHOSPHATIDYL[2-14C]ETHANOLAMINE,1,2-DIOLEOYL](https://img.chemicalbook.in/StructureFile/ChemBookStructure4/GIF/CB6717643.gif)



You may like
-
 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine CAS 4004-05-1View Details
1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine CAS 4004-05-1View Details
4004-05-1 -
 DOPE CAS 4004-05-1View Details
DOPE CAS 4004-05-1View Details
4004-05-1 -
 18:1 (Δ9-Cis) PE (DOPE) CAS 4004-05-1View Details
18:1 (Δ9-Cis) PE (DOPE) CAS 4004-05-1View Details
4004-05-1 -
 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine CAS 4004-05-1View Details
1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine CAS 4004-05-1View Details
4004-05-1 -
 L-α-Phosphatidylethanolamine, dioleoyl CAS 4004-05-1View Details
L-α-Phosphatidylethanolamine, dioleoyl CAS 4004-05-1View Details
4004-05-1 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8