1,1,3,3-Tetramethyldisilazane
Synonym(s):TMDS
- CAS NO.:15933-59-2
- Empirical Formula: C4H15NSi2
- Molecular Weight: 133.34
- MDL number: MFCD00025626
- EINECS: 240-072-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
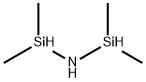
What is 1,1,3,3-Tetramethyldisilazane?
Chemical properties
Clear colorless to faintly yellow liquid
Physical properties
bp 99–100 °C; n20 D 1.4040; d 0.752 g cm?3.
The Uses of 1,1,3,3-Tetramethyldisilazane
1,1,3,3-Tetramethyldisilazane is widely used in intramolecular hydrosilation of allyl alcohols, homoallyl alcohols, and homopropargyl alcohols for the regio- and stereoselective synthesis of polyols
The Uses of 1,1,3,3-Tetramethyldisilazane
Tetramethyldisilazane is used as a gas chromatographic derivatizing reagent. Further, it reacts with phenol to prepare dimethylphenoxysilane. In addition, it is used in electronic, polymer and pharmaceutical industries.
Properties and Applications
The ICP CVD synthesis of SiCxNy: H thin films using 1,1,3,3-tetramethyldisilazane (TMDSZ ) as a single-source precursor and argon as a gas-activator[1]. Using TMDSZ as the single-source compound produced amorphous hydrogenated silicon carbonitride (a-SiCN: H) films, whereas no such films were formed when HMDSZ was used, which was attributed to the lack of Si–H bond in HMDSZ. Under the collision-free condition, the formation of ?CH3, NH3, and DMSA was demonstrated from the decomposition of TMDSZ on the heated W filament. Free-radical short-chain reactions dominate the secondary gas-phase reactions of TMDSZ in the reactor setup. The short-chain reaction is initiated by the formation of methyl radicals via the cleavage of the Si–CH3 bonds of TMDSZ. The H abstraction by the produced methyl radicals from the Si–H or the C–H bond in various stable molecules (e.g., TMDSZ) propagates the chain with a resulting radical, which recombines with another radical to terminate the chain reactions, producing a series of products in the high-mass region[2].
References
[1] Maksim N. Chagin. “Synthesis, Properties and Aging of ICP-CVD SiCxNy:H Films Formed from Tetramethyldisilazane.” Coatings (2022).
[2] James Michael Stevenson, Eric Ampong and Yujun Shi*. “Understanding the Reaction Chemistry of 1,1,3,3-Tetramethyldisilazane as a Precursor Gas in a Catalytic Chemical Vapor Deposition Process.” The Journal of Physical Chemistry A 127 44 (2023): 9185–9195.
Properties of 1,1,3,3-Tetramethyldisilazane
| Melting point: | 99-100 °C |
| Boiling point: | 99-100 °C |
| Density | 0.752 g/mL at 25 °C(lit.) |
| refractive index | n |
| Flash point: | 17 °F |
| storage temp. | Store below +30°C. |
| solubility | Miscible with common organic solvents. |
| form | clear liquid |
| pka | 9.80±0.70(Predicted) |
| Specific Gravity | 0.752 |
| color | Colorless to Almost colorless |
| Sensitive | Moisture Sensitive |
| Hydrolytic Sensitivity | 7: reacts slowly with moisture/water |
| BRN | 741869 |
| CAS DataBase Reference | 15933-59-2(CAS DataBase Reference) |
| EPA Substance Registry System | Silanamine, N-(dimethylsilyl)-1,1-dimethyl- (15933-59-2) |
Safety information for 1,1,3,3-Tetramethyldisilazane
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 1,1,3,3-Tetramethyldisilazane
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid Fmoc-Val-Cit-PAB DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonateRelated products of tetrahydrofuran

You may like
-
 1,1,3,3-Tetramethyldisilazane CAS 15933-59-2View Details
1,1,3,3-Tetramethyldisilazane CAS 15933-59-2View Details
15933-59-2 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8