1,1'-Thiobisethane
Synonym(s):1,1′-Thiobisethane;Ethyl sulfide;NSC 75157
- CAS NO.:352-93-2
- Empirical Formula: C4H10S
- Molecular Weight: 90.19
- MDL number: MFCD00009270
- EINECS: 206-526-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
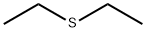
What is 1,1'-Thiobisethane?
Chemical properties
colourless liquid
Occurrence
Reported found in cabbage, sauerkraut, mustard, egg, chicken, beef, pork, beer (0.0002 to 0.0017 ppm), grape brandy (cognac, armagnac, weinbrand).
The Uses of 1,1'-Thiobisethane
Diethyl sulfide is used as a solvent for anhydrous mineral salts and in plating baths for coating metals with gold or silver. It is used as a flavoring agent. It is also used in the addition reaction with platinum dichloride to prepare platinum ethynyl dimers and polymers with pendant ferrocenyl group. Further, it is used in the preparation of triethylsulfoniuim ethylsulfate by reaction with sulfuric acid diethyl ester. In addition to this, it is used in the quantification procedure of volatile sulfur compounds by solid-phase microextraction.
The Uses of 1,1'-Thiobisethane
Diethyl sulfide may be used as an analytical standard for the determination of the analyte in wastewater, wines, and beer by gas chromatography (GC) based techniques.
The Uses of 1,1'-Thiobisethane
Solvent for Anhydrous mineral salts; in plating baths for coating metals with gold or silver.
What are the applications of Application
Diethyl sulfide is a common solvent for anhydrous mineral salts
Definition
ChEBI: An ethyl sulfide compound having two ethyl groups attached to a sulfur atom.
Synthesis Reference(s)
Tetrahedron Letters, 14, p. 3853, 1973 DOI: 10.1016/S0040-4039(01)87056-8
General Description
A colorless oily liquid with a garlic-like odor. Less dense than water. Flash point 20°F. Vapors heavier than air. May irritate skin and eyes. Used to make other chemicals.
Air & Water Reactions
Highly flammable. Slightly soluble in water.
Reactivity Profile
Organosulfides, such as 1,1'-Thiobisethane, are incompatible with acids, diazo and azo compounds, halocarbons, isocyanates, aldehydes, alkali metals, nitrides, hydrides, and other strong reducing agents. Reactions with these materials generate heat and in many cases hydrogen gas. Many of these compounds may liberate hydrogen sulfide upon decomposition or reaction with an acid.
Health Hazard
May cause toxic effects if inhaled or absorbed through skin. Inhalation or contact with material may irritate or burn skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Safety Profile
Mildly toxic by ingestion. A skin and eye irritant. A very dangerous fire hazard when exposed to heat, flame, or sparks; can react vigorously with oxidizers. Reacts with water, steam, acids, or acid fumes to produce toxic and flammable vapors. To fight fire, use water spray or mist, dry chemical, CO2, foam. When heated to decomposition it yields highly toxic fumes of SOx. See also SULFIDES.
Purification Methods
Wash the sulfide with aqueous 5% NaOH, then water, dry with CaCl2 and distil it from sodium. It can also be dried with MgSO4 or silica gel. Alternative purification is via the Hg(II) chloride complex [(Et)2S.2HgCl2] (see dimethyl sulfide). [Beilstein 1 IV 1394.]
Properties of 1,1'-Thiobisethane
| Melting point: | −100 °C(lit.) |
| Boiling point: | 90-92 °C(lit.) |
| Density | 0.837 g/mL at 25 °C(lit.) |
| vapor pressure | 105 mm Hg ( 37.7 °C) |
| refractive index | n |
| FEMA | 3825 | DIETHYL SULFIDE |
| Flash point: | 15 °F |
| solubility | H2O: insoluble |
| form | liquid |
| color | Colorless to Almost colorless |
| Specific Gravity | 0.837 |
| Odor | ethereal sulfurous |
| Odor Threshold | 0.000033ppm |
| Water Solubility | Miscible with alcohol, ethanol and ether. Slightly miscible with carbon tetrachloride. Immiscible with water. |
| Merck | 14,3854 |
| JECFA Number | 454 |
| BRN | 1696909 |
| Dielectric constant | 7.2(20℃) |
| Stability: | Stable. Highly flammable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 352-93-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Diethyl sulfide(352-93-2) |
| EPA Substance Registry System | Diethyl sulfide (352-93-2) |
Safety information for 1,1'-Thiobisethane
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 1,1'-Thiobisethane
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 Ethyl Sulfide CAS 352-93-2View Details
Ethyl Sulfide CAS 352-93-2View Details
352-93-2 -
 Diethyl sulfide CAS 352-93-2View Details
Diethyl sulfide CAS 352-93-2View Details
352-93-2 -
 Diethyl sulfide CAS 352-93-2View Details
Diethyl sulfide CAS 352-93-2View Details
352-93-2 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1