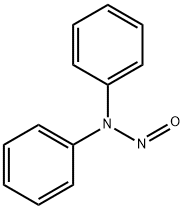
1,1-DIPHENYLHYDRAZINE
- CAS NO.:530-50-7
- Empirical Formula: C12H12N2
- Molecular Weight: 184.24
- MDL number: MFCD00044505
- EINECS: 208-483-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-06-08 17:06:38
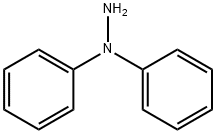
What is 1,1-DIPHENYLHYDRAZINE?
Description
Diphenylhydrazine is a man-made chemical that occurs in two isomeric forms: 1,1-diphenylhydrazine and 1,2-diphenylhdrazine. Diphenylhydrazine is produced by the reduction of nitrobenzene. Little or no information is available for 1,1-diphenylhydrazine. Most toxicological and use data pertain to 1,2-diphenylhydrazine.
The Uses of 1,1-DIPHENYLHYDRAZINE
Previously, 1,2-diphenylhydrazine was used for producing benzidine that was used in the synthesis of benzidine-based dyes. However, these dyes are no longer produced in the United States. The primary use of 1,2-diphenylhydrazine is in the production of the anti-inflammatory agent phenylbutazone and sulfinpyrazone, a uricosuric agent.
Synthesis Reference(s)
The Journal of Organic Chemistry, 33, p. 3963, 1968 DOI: 10.1021/jo01274a068
General Description
Yellow crystals. Insoluble in water.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Toxic gases may be formed by mixing 1,1-DIPHENYLHYDRAZINE with acids, aldehydes, amides, carbamates, cyanides, inorganic fluorides, halogenated organics, isocyanates, ketones, metals, nitrides, peroxides, phenols, epoxides, acyl halides, and strong oxidizing or reducing agents. Flammable gases are formed by mixing materials in this group with alkali metals. Explosive combination can occur with strong oxidizing agents, metal salts, peroxides, and sulfides.
Environmental Fate
Definitive information regarding the environmental fate and behavior of diphenylhydrazine in environmental media is extremely limited. No information is available affirming persistence of diphenylhydrazine in the environment or definitive pathways for its degradation. The low vapor pressure of diphenylhydrazine precludes it being a significant air contaminant. Diphenylhydrazine may be moderately absorbed into soil but it would be quickly oxidized to azobenzene. The half-life of diphenylhydrazine in water is reportedly less than 15 min and is likely due to its oxidation to azobenzene and benzidine.
Purification Methods
Purify it via the hydrochloride [530-47-2], which has m 165-170o(dec) after crystallization from aqueous EtOH (+ a few drops of HCl) and recover the free base with aqueous NaOH, extract it into Et2O, dry it (KOH), filter, evaporate and distil the residue under a vacuum. The distillate crystallises on cooling. The benzoyl derivative has m 194o (from Me2CO), and the 4-nitrophenyl hydrazone (with the aldehyde) has m 131-132o. [Koga & Anselme J Org Chem 33 3963 1968, Beilstein 15 IV 55.]
Toxicity evaluation
The mechanism of action of diphenylhydrazine is not known. It is possible that some toxic effects may be attributed to its major metabolites, aniline and azobenzene, both of which are known carcinogens. Results of metabolism studies reporting aniline, benzidine, hyroxybenzidines, and aminophenols as metabolites in rats following multiple routes of administration suggest that the diphenylhydrazine metabolism may be similar to that of azobenzene and aniline. It is also possible that conversion of 1,2-diphenylhydrazine to aniline in the gastrointestinal tract may occur due to intestinal microflora and via acid hydrolysis.
Properties of 1,1-DIPHENYLHYDRAZINE
| Melting point: | 50.5°C |
| Boiling point: | 308.2°C (rough estimate) |
| Density | 1,12 g/cm3 |
| refractive index | 1.6266 (estimate) |
| pka | 3.34±0.10(Predicted) |
| form | powder to lump to clear liquid |
| color | White or Colorles to Yellow to Orange |
| Merck | 14,3325 |
| CAS DataBase Reference | 530-50-7(CAS DataBase Reference) |
| EPA Substance Registry System | 1,1-Diphenylhydrazine (530-50-7) |
Safety information for 1,1-DIPHENYLHYDRAZINE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P501:Dispose of contents/container to..… |
Computed Descriptors for 1,1-DIPHENYLHYDRAZINE
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
![1-[4-(Diethylamino)benzylidene]-2,2-diphenylhydrazine](https://img.chemicalbook.in/CAS/GIF/68189-23-1.gif)

You may like
-
 1,1-Diphenylhydrazine CAS 530-50-7View Details
1,1-Diphenylhydrazine CAS 530-50-7View Details
530-50-7 -
 1,1-Diphenylhydrazine CASView Details
1,1-Diphenylhydrazine CASView Details -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4