530-62-1
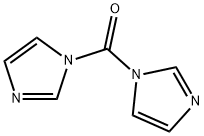
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid; [Merck Index] White crystalline powder; [Alfa Aesar MSDS] |
|---|---|
| Chemical Classes | Nitrogen Compounds -> Imidazoles |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 162.15 g/mol |
|---|---|
| XLogP3 | -0.2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 162.05416083 g/mol |
| Monoisotopic Mass | 162.05416083 g/mol |
| Topological Polar Surface Area | 52.7 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 166 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
uses
1,1′-Carbonyldiimidazole (CDI) can be used:
In the activation of malonic acid derivatives to prepare carbonyl imidazoles by mild decarboxylation.
In the activation of cellulose membranes for the development of immunosensors.
In the synthesis of substituted 1,3-oxazolo[4,5-d] pyridazinones.
As a reagent for the conversion of various hydroxamic acids to isocyanates by Lossen rearrangement.
In the synthesis of 1,3,4-oxadiazole derivatives [6], and peptide thioesters in water.
As an acylation agent for the synthesis of carbamates.
Description
1,1'-Carbonyldiimidazole is a reagent widely utilized in organic synthesis, particularly for the activation of carboxylic acids to form amides, esters, and anhydrides. It is renowned for its efficiency in facilitating the formation of peptide bonds, which is a central reaction in the synthesis of polypeptides and proteins in the laboratory. Researchers employ 1,1'-Carbonyldiimidazole in the preparation of various acylated compounds, taking advantage of its ability to act as a coupling agent that minimizes the formation of side products. This reactivity is also exploited in the synthesis of heterocycles, where it is used to introduce carbonyl groups into cyclic frameworks. Within the realm of material science, it is studied for its role in modifying the surface chemistry of polymers, where it can be used to attach bioactive molecules or other functional groups to polymer backbones.
