1-Nitropropane
Synonym(s):1-Nitropropane;N-Nitropropane
- CAS NO.:108-03-2
- Empirical Formula: C3H7NO2
- Molecular Weight: 89.09
- MDL number: MFCD00007407
- EINECS: 203-544-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:47
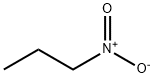
What is 1-Nitropropane ?
Description
1-Nitropropane (1-NP), a representative small nitro-alkane, is widely used as an industrial solvent and a fuel additive in automobiles and aircraft engines. The improvement effects of 1-NP on fuel combustion demonstrate that 1-NP served as an initiator can rapidly produce a large number of reactive radicals, which significantly increases the fuel conversion rate. In addition, among the small nitro-alkanes, the molecular structure of 1-NP endows it with a higher specific impulse. It provides the possibilities for its application as propellant in long-range rocket systems[1].
Chemical properties
Colorless liquid.Soluble in water 1.4 mL/100 mL (20C); solubility of water in 1-nitropropane 0.5 cc/100 cc (20C).
Chemical properties
1-Nitropropane is a colorless liquid with a mild, fruity odor.
Physical properties
Colorless, oily liquid with a mild, fruity odor. A detection odor threshold concentration of 510 mg/m3 (140 ppmv) was experimentally determined by Dravnieks (1974).
The Uses of 1-Nitropropane
The solvent of 1-Nitropropane is mainly used coatings, inks and separation processes.
The Uses of 1-Nitropropane
Solvent, chemical synthesis, rocket propellant, gasoline additive.
The Uses of 1-Nitropropane
Solvent for organic materials; propellant fuel; gasoline additive
Production Methods
1-Nitropropane is produced by vapor-phase nitration of propane with nitric acid (Baker and Bollmeier 1978) or by the vapor-phase nitration of butanol (HSDB 1988).
Definition
ChEBI: 1-nitropropane is a nitroalkane that is propane substituted at C-1 by a nitro group. It derives from a hydride of a propane.
Hazard
Flammable, moderate fire risk, moderate explosion hazard when shocked or heated. Liver damage, eye and upper respiratory tract irritant. Questionable carcinogen.
Health Hazard
Humans exposed briefly to vapors of 1-nitropropane were found to have concentrations exceeding 100 p.p.m. irritating to the eyes (HSDB 1988). The chemical may produce anorexia, nausea, vomiting, diarrhea as well as injury to the liver and kidneys in exposed humans. High concentrations of the chemical also may produce methemoglobinemia with cyanosis. 1-Nitropropane vapors also are damaging to the lungs. 1-Nitropropane is, however, more acutely irritating to mucus membranes than 2-nitropropane. Despite this, the TLV for human exposure to 2-nitropropane is less than that for 1-nitropropane, based on the carcinogenic potential of 2-nitropropane in rats.
Industrial uses
1-Nitropropane is used as a solvent for cellulose acetate, vinyl resins and lacquers. It is also used as a gasoline additive (HSDB 1988).
Safety Profile
Poison by ingestion and intraperitoneal routes. Mildly toxic by inhalation. A human eye irritant. Human systemic effects by inhalation: conjunctiva irritation. Mutation data reported. Very dangerous fire hazard when exposed to heat, open flame, or oxidizers. Reacts violently with Ca(OH)2, hydrocarbons, hydroxides, inorganic bases. May explode on heating. Metal oxides increase its sensitivity to thermal ignition. To fight fire, use alcohol foam, CO2, dry chemical, water spray. When heated to decomposition it emits toxic fumes of NOx. See also 2- NITROPROPANE, NITROALKANES, and NITRO COMPOUNDS.
Potential Exposure
1-Nitropropane is used as a solvent for polymers, as a stabilizer; and in organic synthesis. Note: Technical products measurably contaminated with 2-Nitropropane, see also 2-Nitropropane (N: 0550)
Metabolism
1-Nitropropane is a substrate for the liver microsomal cytochrome P-450-dependent
mixed-function oxidase system. Oxidative denitrification of 1-nitropropane by
phenobarbital-induced rat microsomes occurred at a rate of 0.6 nmole/min/mg
protein. This rate was much slower than for 2-nitropropane which was metabolized
at 2.4 nmole/min/mg microsomal protein (Ullrich et al 1978).
The role of the cytochrome P-450 system in vivo in 1-nitropropane metabolism
is unknown. Bray and James (1958) isolated a small amount of a mercapturic acid
metabolite from the urine of rabbits dosed with 1-nitropropane.
Shipping
UN2608 Nitropropanes, Hazard Class: 3; Labels: 3-Flammable liquid.
Purification Methods
Purify it as for nitromethane. [Beilstein 1 IV 229.]
Incompatibilities
1-Nitropropane, a nitroparaffinin, forms exposive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, reducing agents; nitrates, amines, hydrocarbons, and other combustible materials; metal oxides. May explode on heating.
References
[1] Li He . “Ignition characteristics of 1-Nitropropane: Experimental measurements and kinetic modeling.” Fuel 317 (2022): Article 123385.
Properties of 1-Nitropropane
| Melting point: | -108 °C |
| Boiling point: | 132 °C |
| Density | 0.998 g/mL at 25 °C(lit.) |
| vapor density | 3.1 (vs air) |
| vapor pressure | 7.5 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 93 °F |
| storage temp. | Store below +30°C. |
| solubility | 14g/l |
| form | Liquid |
| pka | pK1:8.98 (25°C) |
| color | Clear |
| PH | 6.0 (0.9g/l, H2O, 20℃) |
| explosive limit | 2.2-11.0%(V) |
| Water Solubility | 1.40 g/100 mL |
| Merck | 14,6626 |
| BRN | 506236 |
| Henry's Law Constant | 6.11 at 25 °C (static headspace-GC, Welke et al., 1998) |
| Exposure limits | NIOSH REL: TWA 25 ppm (90 mg/m3), IDLH 1,000 ppm; OSHA PEL: TWA
25 ppm; ACGIH TLV: TWA 25 ppm (adopted). |
| Dielectric constant | 23.239999999999998 |
| Stability: | Stable. Flammable. Incompatible with strong bases, strong oxidizing agents. |
| CAS DataBase Reference | 108-03-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Propane, 1-nitro-(108-03-2) |
| EPA Substance Registry System | 1-Nitropropane (108-03-2) |
Safety information for 1-Nitropropane
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H226:Flammable liquids H331:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. |
Computed Descriptors for 1-Nitropropane
| InChIKey | JSZOAYXJRCEYSX-UHFFFAOYSA-N |
1-Nitropropane manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 1-Nitropropane, 98% CAS 108-03-2View Details
1-Nitropropane, 98% CAS 108-03-2View Details
108-03-2 -
 1-Nitropropane CAS 108-03-2View Details
1-Nitropropane CAS 108-03-2View Details
108-03-2 -
 1-Nitropropane CAS 108-03-2View Details
1-Nitropropane CAS 108-03-2View Details
108-03-2 -
 1-Nitropropane CAS 108-03-2View Details
1-Nitropropane CAS 108-03-2View Details
108-03-2 -
 1-Nitropropane CAS.108-03-2View Details
1-Nitropropane CAS.108-03-2View Details
108-03-2 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
