1-Methylnaphthalene
Synonym(s):α-Methylnaphthalene;1-Methylnaphthalene;1-MN
- CAS NO.:90-12-0
- Empirical Formula: C11H10
- Molecular Weight: 142.2
- MDL number: MFCD00004034
- EINECS: 201-966-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-10 11:56:18
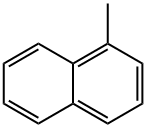
What is 1-Methylnaphthalene?
Description
1-methylnaphthalene is a methylnaphthalene carrying a methyl substituent at position. It is a polycyclic aromatic hydrocarbon (PAH). It is present in cigarette smoke, wood smoke, tar, asphalt, and at some hazardous waste sites. The main use of 1-methylnaphthalene is as a raw material for naphthoic acid, fluorescent whitening agents, and surfactants. It is also used as a raw material for dyestuff dispersants and heat transfer oils, and as a solvent for agricultural chemical.
Chemical properties
1-methylnaphthalene is a colorless, bluefluorescing liquid with an earthy, phenolic odor. It is insoluble in water but dissolves in alcohol and ether. With a density greater than water, this combustible substance is obtained from coal tar and used in organic synthesis. It has a role as a carcinogenic agent and a plant metabolite.
Occurrence
Methylnaphthalene was identified as a volatile component of cassava, roasted filberts and nectarines. Assorted types of lima, pinto, red kidney, black, navy and mung beans, soybeans, split peas and lentils were found to contain 1-methylnaphthalene at concentrations ranging from 2.8 to 49.2 ppb.
The Uses of 1-Methylnaphthalene
The main uses of methylnaphthalene are as a raw material for dyestuff dispersants and heat transfer oils, and as a solvent for agricultural chemical. It is used in insecticide manufacturing; manufacture of phthalic anhydride; solvent in organic synthesis; asphalt and naptha constituent. It is also used as a test substance for the determination of the cetane number of diesel fuels. Further, it is employed in the preparation of 1-methylnaphthalene-d10 using deuterium oxide, sodium deuteroxide.
Preparation
1-Methylnaphthalene is primarily derived from coal tar and petroleum oils. It is present in high-temperature coal tar in a concentration of 0.5% and is produced industrially from the methylnaphthalene fraction, which boils between 240 and 245 °C, by redistillation of the 2-methylnaphthalene filtrate following crystallization and separation of 2-methylnaphthalene.
Definition
ChEBI: 1-methylnaphthalene is a methylnaphthalene carrying a methyl substituent at position 1. It has a role as a carcinogenic agent and a plant metabolite.
Aroma threshold values
Detection: 7.5 to 20 ppb.
Taste threshold values
Taste characteristics at 1 ppm: naphthyl-like with a medicinal nuance.
Synthesis Reference(s)
Journal of the American Chemical Society, 65, p. 295, 1943 DOI: 10.1021/ja01242a503
The Journal of Organic Chemistry, 53, p. 4466, 1988 DOI: 10.1021/jo00254a009
Tetrahedron Letters, 29, p. 97, 1988 DOI: 10.1016/0040-4039(88)80026-1
General Description
1-methylnaphthalene is a colorless liquid. Freezing point -22 °C (7.6 °F). Boiling point 240-243 °C (464-469 °F). Flash point 82 °C (180 °F). Denser than water. Derived from coal tar and used in organic synthesis.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
1-Methylnaphthalene is sensitive to heat. Reacts with strong oxidizing agents. Incompatible with oxygen and peroxides .
Hazard
Moderate fire risk. Lower respiratory tractirritant and lung damage. Questionable carcinogen.
Health Hazard
Harmful if inhaled. Liquid causes irritation of the eyes and skin and skin photosensitization. Harmful if swallowed. Chronic exposure may cause liver or kidney damage.
Fire Hazard
1-Methylnaphthalene is combustible.
Carcinogenicity
The carcinogenic potential of 1- and 2-methyl was investigated in B6C3F1 mice. Female and male mice were given methylnaphthalene in their diets for 81 weeks. The results indicated that 1-methyl was a possible weak carcinogen in the lung of male but not female mice whereas 2-methyl did not possess unequivocal carcinogenic potential in these mice.
Purification Methods
Dry 1-methylnaphthalene for several days with CaCl2 or by prolonged refluxing with BaO. Fractionally distil it through a glass helices-packed column from sodium. Purify it further by solution in MeOH and precipitation of its picrate complex by adding to a saturated solution of picric acid in MeOH. The picrate, after crystallisation to constant melting point (m 140-141o) from MeOH, is dissolved in *benzene and extracted with aqueous 10% LiOH until the extract is colourless. Evaporation of the *benzene solution under vacuum gives 1-methylnaphthalene [Kloetzel & Herzog J Am Chem Soc 72 1991 1950]. However, neither the picrate nor the styphnate complexes satisfactorily separate 1-and 2-methylnaphthalenes. To achieve this, 2-methylnaphthalene (10.7g) in 95% EtOH (50mL) has been precipitated with 1,3,5-trinitrobenzene (7.8g) and this complex has been crystallised from MeOH to m 153-153.5o (m of the 2-methyl isomer is 124o). [Alternatively, 2,4,7-trinitrofluorenone in hot glacial acetic acid could be used, and the derivative (m 163-164o) is recrystallised from glacial acetic acid]. The 1-methylnaphthalene is regenerated by passing a soution of the complex in dry *benzene through a 15-in column of activated alumina and washing with *benzene/pet ether (b 35-60o) until the coloured band of the nitro compound had moved down near the end of the column. The complex can also be decomposed using tin and acetic-hydrochloric acids, followed by extraction with diethyl ether and *benzene; the extracts are washed successively with dilute HCl, strongly alkaline sodium hypophosphite, water, dilute HCl and water. [Soffer & Stewart J Am Chem Soc 74 567 1952.] It can be freed from anthracene by zone melting [Beilstein 5 IV 1687.]
Properties of 1-Methylnaphthalene
| Melting point: | −22 °C(lit.) |
| Boiling point: | 240-243 °C(lit.) |
| Density | 1.001 g/mL at 25 °C(lit.) |
| vapor pressure | 2 hPa (25 °C) |
| FEMA | 3193 | 1-METHYLNAPHTHALENE |
| refractive index | n |
| Flash point: | 180 °F |
| storage temp. | Store below +30°C. |
| solubility | 0.026g/l |
| form | Liquid |
| pka | 37(at 25℃) |
| color | Clear colorless to yellow |
| Odor | at 1.00 % in dipropylene glycol. naphthyl chemical medicinal camphor |
| explosive limit | 0.7-6.5%(V) |
| Water Solubility | 0.026 g/L (25 ºC) |
| JECFA Number | 1335 |
| BRN | 506793 |
| Exposure limits | ACGIH: TWA 0.5 ppm (Skin) |
| Dielectric constant | 2.7(20℃) |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents, oxygen. |
| CAS DataBase Reference | 90-12-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Naphthalene, 1-methyl-(90-12-0) |
| EPA Substance Registry System | 1-Methylnaphthalene (90-12-0) |
Safety information for 1-Methylnaphthalene
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H304:Aspiration hazard H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. |
Computed Descriptors for 1-Methylnaphthalene
| InChIKey | QPUYECUOLPXSFR-UHFFFAOYSA-N |
1-Methylnaphthalene manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 90-12-0 1-Methylnaphthalene, 96% 99%View Details
90-12-0 1-Methylnaphthalene, 96% 99%View Details
90-12-0 -
 1-Methylnaphthalene CAS 90-12-0View Details
1-Methylnaphthalene CAS 90-12-0View Details
90-12-0 -
 1-Methylnaphthalene CAS 90-12-0View Details
1-Methylnaphthalene CAS 90-12-0View Details
90-12-0 -
 1-Methylnaphthalene CAS 90-12-0View Details
1-Methylnaphthalene CAS 90-12-0View Details
90-12-0 -
 1-Methylnaphthalene CAS 90-12-0View Details
1-Methylnaphthalene CAS 90-12-0View Details
90-12-0 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
