β-Caryophyllene
Synonym(s):β-Caryophyllene;(−)-trans-Caryophyllene;trans-(1R,9S)-8-Methylene-4,11,11-trimethylbicyclo[7.2.0]undec-4-ene
- CAS NO.:87-44-5
- Empirical Formula: C15H24
- Molecular Weight: 204.35
- MDL number: MFCD00075925
- EINECS: 201-746-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
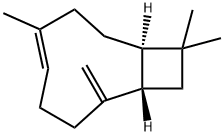
What is β-Caryophyllene?
Description
β-Caryophyllene (Item No. 21572) is a sesquiterpene that has been found in plants, including C. sativa, C. indica, and hemp, and has diverse biological activities, including lipid metabolic, antioxidant, anti-neuroinflammatory, anti-proliferative, and antinociceptive properties. It is an agonist of the cannabinoid (CB) receptor CB2 (Ki = 155 nM) that inhibits cAMP production induced by forskolin in CHO-K1 cells expressing CB2 receptors (EC50 = 38 nM). β-Caryophyllene is also an agonist of peroxisome proliferator-activated receptor α (PPARα; EC50 = 9.58 μM in a reporter assay). β-Caryophyllene (1 and 2.5 μM) reduces the production of reactive oxygen species (ROS) in and protects against cytotoxicity of SH-SY5Y cells induced by 1-methyl-4-pheylpyridinium (MPP+). It also decreases the β-amyloid burden in the hippocampus and cerebral cortex and improves memory in an APP/PS1 transgenic mouse model of Alzheimer’s disease, decreasing the latency to find the platform in the Morris water maze during training and increasing the time spent in the target quadrant during testing when administered at a dose of 48 mg/kg per day. β-Caryophyllene (50 mg/kg) increases the number of entries into and the time spent in the open arms of the elevated plus maze and the time spent immobile in the forced swim test, indicating anxiolytic-like and antidepressant-like activity, effects that can be blocked by the CB2 receptor antagonist AM630 .
Chemical properties
Clear colorless liquid
Chemical properties
β-Caryophyllene has a woody-spicy, dry, clove-like aroma.
Occurrence
Three isomers (α-, β-, and γ-caryophyllene) are found in nature. The β-isomer is the most frequently encountered and most abundant. This sesquiterpene hydrocarbon occurs naturally in approximately 60 essential oils, mainly in that of cloves, from which it was originally isolated. The chemical structure has been thoroughly studied; other studies have been conducted on the isolation and the dipolar moment, as well as on its oxide. Reported found in lime peel oil, lemon, grapefruit, guava fruit, raspberry, black currant, carrot, celery seed, cinnamon bark, anise, nutmeg, cumin seed, ginger, pepper, peppermint oil, mace, laurel and caraway herb.
The Uses of β-Caryophyllene
β-Caryophyllene is notable for having a cyclobutane ring, a rarity in nature. β-Caryophyllene is one of the chemical compounds that contributes to the spiciness of black pepper. β-Caryophyllene was shown to selectively bind to the cannabinoid receptor type-2 (CB2) and to exert significant cannabimimetic antiinflammatory effects in mice.
What are the applications of Application
(?)-trans-Caryophyllene is a phytochemical compound that is a constituent of many essential oils
Definition
ChEBI: A beta-caryophyllene in which the stereocentre adjacent to the exocyclic double bond has S configuration while the remaining stereocentre has R configuration. It is the most commonly occurring form of <greek beta-caryophyllene, occurring in many essential oils, particularly oil of cloves.
Preparation
Isolated from oil of clove stems and separated from eugenol by treating the oil with 7% sodium carbonate solution, extracting with ether, repeating the carbonate treatment on the concentrated extracts, and finally steam distilling.
Aroma threshold values
Detection at 64 to 90 ppb
Taste threshold values
Taste characteristics at 50 ppm: spicy, pepper-like, woody, camphoraceous with a citrus background.
Synthesis Reference(s)
Journal of the American Chemical Society, 86, p. 485, 1964 DOI: 10.1021/ja01057a040
General Description
Pale yellow oily liquid with an odor midway between odor of cloves and turpentine.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
The unsaturated aliphatic hydrocarbons, such as BETA-CARYOPHYLLENE, are generally much more reactive than the alkanes. Strong oxidizers may react vigorously with them. Reducing agents can react exothermically to release gaseous hydrogen. In the presence of various catalysts (such as acids) or initiators, compounds in this class can undergo very exothermic addition polymerization reactions.
Fire Hazard
BETA-CARYOPHYLLENE is combustible.
Safety Profile
A skin irritant. Combustible liquid. When heated to decomposition it emits acrid smoke and irritating fumes.
Carcinogenicity
Caryophyllene showed significant activity as an inducer of the detoxifying enzyme glutathione S-transferase in the mouse liver and small intestine. The ability of natural anticarcinogens to induce detoxifying enzymes has been found to correlate with their activity in the inhibition of chemical carcinogenesis (253a).
Properties of β-Caryophyllene
| Melting point: | <25℃ |
| Boiling point: | 129-130 °C/14 mmHg (lit.) |
| alpha | D -8 to -9° (chloroform) |
| Density | 0.901 g/mL at 25 °C (lit.) |
| vapor pressure | 6Pa at 20℃ |
| refractive index | n |
| FEMA | 2252 | BETA-CARYOPHYLLENE |
| Flash point: | 205 °F |
| storage temp. | -20°C |
| solubility | Chloroform (Sparingly), DMSO (Slightly), Methanol (Slightly) |
| form | neat |
| form | Liquid |
| color | Colourless |
| Specific Gravity | 0.90 |
| Odor | at 100.00 %. sweet woody spice clove dry |
| optical activity | [α]23/D 7.5°, neat |
| Water Solubility | 88μg/L at 20℃ |
| JECFA Number | 1324 |
| Merck | 14,1875 |
| BRN | 2044564 |
| Stability: | Light Sensitive |
| CAS DataBase Reference | 87-44-5(CAS DataBase Reference) |
| EPA Substance Registry System | Bicyclo[7.2.0]undec-4-ene, 4,11,11-trimethyl-8-methylene-, (1R,4E,9S)- (87-44-5) |
Safety information for β-Caryophyllene
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H304:Aspiration hazard H317:Sensitisation, Skin |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P272:Contaminated work clothing should not be allowed out of the workplace. P280:Wear protective gloves/protective clothing/eye protection/face protection. P331:Do NOT induce vomiting. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for β-Caryophyllene
β-Caryophyllene manufacturer
Neeru Menthol Pvt Ltd
Ghaziabad Aromatics
Indenta Chemicals (India) Pvt Ltd
Arpan Aromatics
Aamirav Ingredients And Specialties Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran





You may like
-
 Beta-Caryophyllene 87-44-5 98%View Details
Beta-Caryophyllene 87-44-5 98%View Details
87-44-5 -
 Beta-Caryophyllene 87-44-5 98%View Details
Beta-Caryophyllene 87-44-5 98%View Details
87-44-5 -
 beta-Caryophyllene 98% (sum of enantiomers CAS 87-44-5View Details
beta-Caryophyllene 98% (sum of enantiomers CAS 87-44-5View Details
87-44-5 -
 β-Caryophyllene CAS 87-44-5View Details
β-Caryophyllene CAS 87-44-5View Details
87-44-5 -
 (−)-trans-Caryophyllene CAS 87-44-5View Details
(−)-trans-Caryophyllene CAS 87-44-5View Details
87-44-5 -
 beta-Caryophyllene solution CAS 87-44-5View Details
beta-Caryophyllene solution CAS 87-44-5View Details
87-44-5 -
 (−)-trans-Caryophyllene CAS 87-44-5View Details
(−)-trans-Caryophyllene CAS 87-44-5View Details
87-44-5 -
 (−)-trans-Caryophyllene CAS 87-44-5View Details
(−)-trans-Caryophyllene CAS 87-44-5View Details
87-44-5