ε-Caprolactone
Synonym(s):2-Oxepanone;ε-Caprolactone;gamma-Caprolactone;6-Hexanolactone;6-Caprolactone monomer
- CAS NO.:502-44-3
- Empirical Formula: C6H10O2
- Molecular Weight: 114.14
- MDL number: MFCD00003267
- EINECS: 207-938-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-09 10:27:52
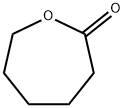
What is ε-Caprolactone?
Chemical properties
Clear colorless liquid, yellowing on ageing
The Uses of ε-Caprolactone
ε-Caprolactone is widely used as a monomer in the manufacturing of highly specialized polymers. It is mainly utilized as a precursor to caprolactam. It finds an application in the synthesis of polyglecaprone, which is used as a suture material in surgery.
The Uses of ε-Caprolactone
Intermediate in adhesives, urethane coatings, and elastomers; solvent; diluent for epoxy resins; synthetic fibers; organic synthesis.
The Uses of ε-Caprolactone
ε-caprolactone is popularly used in the preparation of poly caprolactone (PCL).
What are the applications of Application
ε-Caprolactone is a lactone building block used for the preparation of titanium alkoxides based on benzotriazole phenoxide ligands
Definition
ChEBI: A epsilon-lactone that is oxepane substituted by an oxo group at position 2.
Synthesis Reference(s)
Journal of the American Chemical Society, 80, p. 4079, 1958 DOI: 10.1021/ja01548a063
The Journal of Organic Chemistry, 61, p. 4560, 1996 DOI: 10.1021/jo952237x
Tetrahedron Letters, 17, p. 2455, 1976 DOI: 10.1016/0040-4039(76)90018-6
General Description
ε-caprolactone is a cyclic ester. Ring opening polymerization of ε-caprolactone initiated by, tributylin n butoxide, ethyl magnesium bromide, or samarium acetate or heteropolyacid, leads to the formation of poly caprolactone (PCL).
Flammability and Explosibility
Not classified
Properties of ε-Caprolactone
| Melting point: | -1 °C |
| Boiling point: | 96-97.5 °C10 mm Hg(lit.) |
| Density | 1.03 g/mL at 25 °C(lit.) |
| vapor density | 3.9 (vs air) |
| vapor pressure | 0.01 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 229 °F |
| storage temp. | Store below +30°C. |
| solubility | Chloroform, Ethyl Acetate |
| form | Liquid |
| color | Clear colorless, yellowing on aging |
| explosive limit | 1.2-9%(V) |
| Water Solubility | >1000 MG/L (20 ºC) |
| BRN | 106919 |
| CAS DataBase Reference | 502-44-3(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Oxepanone(502-44-3) |
| EPA Substance Registry System | 2-Oxepanone (502-44-3) |
Safety information for ε-Caprolactone
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for ε-Caprolactone
| InChIKey | PAPBSGBWRJIAAV-UHFFFAOYSA-N |
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran



You may like
-
 ε-Caprolactone monomer CAS 502-44-3View Details
ε-Caprolactone monomer CAS 502-44-3View Details
502-44-3 -
 epsilon-Caprolactone monomer >99% (GC) CAS 502-44-3View Details
epsilon-Caprolactone monomer >99% (GC) CAS 502-44-3View Details
502-44-3 -
 Epsilon-caprolactone 98% CAS 502-44-3View Details
Epsilon-caprolactone 98% CAS 502-44-3View Details
502-44-3 -
 ε-Caprolactone, 97% CAS 502-44-3View Details
ε-Caprolactone, 97% CAS 502-44-3View Details
502-44-3 -
 ε-Caprolactone CAS 502-44-3View Details
ε-Caprolactone CAS 502-44-3View Details
502-44-3 -
 Dimethyl adipate 95% CAS 502-44-3View Details
Dimethyl adipate 95% CAS 502-44-3View Details
502-44-3 -
 ε-Caprolactone CAS 502-44-3View Details
ε-Caprolactone CAS 502-44-3View Details
502-44-3 -
 ε-Caprolactone CAS 502-44-3View Details
ε-Caprolactone CAS 502-44-3View Details
502-44-3
