989-51-5
Product Name:
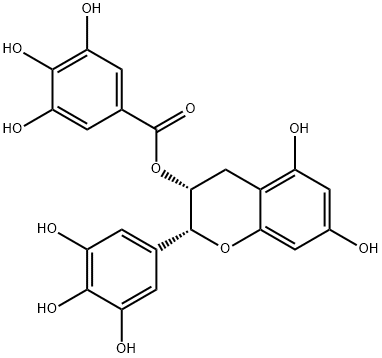
(-)-Epigallocatechin gallate
Formula:
C22H18O11
Synonyms:
EGCG;(?)-cis-2-(3,4,5-Trihydroxyphenyl)-3,4-dihydro-1(2H)-benzopyran-3,5,7-triol 3-gallate;(?)-cis-3,3′,4′,5,5′,7-Hexahydroxy-flavane-3-gallate;(?)-Epigallocatechin gallate
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid |
|---|---|
| Melting Point | 140 - 142 °C |
| Collision Cross Section | 206.1 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards] |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 458.4 g/mol |
|---|---|
| XLogP3 | 1.2 |
| Hydrogen Bond Donor Count | 8 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 4 |
| Exact Mass | 458.08491139 g/mol |
| Monoisotopic Mass | 458.08491139 g/mol |
| Topological Polar Surface Area | 197 Ų |
| Heavy Atom Count | 33 |
| Formal Charge | 0 |
| Complexity | 667 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
(-)-epigallocatechin 3-gallate is a gallate ester obtained by the formal condensation of gallic acid with the (3R)-hydroxy group of (-)-epigallocatechin. It has a role as an antineoplastic agent, an antioxidant, a Hsp90 inhibitor, a neuroprotective agent, a plant metabolite, a geroprotector and an apoptosis inducer. It is a gallate ester, a polyphenol and a member of flavans. It is functionally related to a (-)-epigallocatechin.
