CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Hexachlorobutadiene appears as a colorless liquid with a mild odor. Insoluble in water and denser than water. Nonflammable. May be toxic by ingestion or inhalation. Used as a solvent and heat transfer fluid. |
|---|---|
| Color/Form | Clear, colorless liquid |
| Odor | Mild, turpentine-like odor. |
| Boiling Point | 419 °F at 760 mmHg (NTP, 1992) |
| Melting Point | -6 °F (NTP, 1992) |
| Flash Point | 90 °C |
| Solubility | less than 0.1 mg/mL at 72 °F (NTP, 1992) |
| Density | 1.675 at 59.9 °F (USCG, 1999) - Denser than water; will sink |
| Vapor Density | 8.99 (NTP, 1992) - Heavier than air; will sink (Relative to Air) |
| Vapor Pressure | 0.15 mmHg at 68 °F ; approximately 0.3 mmHg at 77 °F (NTP, 1992) |
| LogP | log Kow = 4.78 |
| Henry's Law Constant | Henry's Law constant = 1.03X10-2 atm-cu m/mole @ 20 °C |
| Autoignition Temperature | 1130 °F (USCG, 1999) |
| Decomposition | When heated to decomposition it emits very toxic fumes of /chlorine/ Cl-. |
| Viscosity | 2.447 centipoise at 37.7 °C, 1.479 centistokes; 1.131 centipoise at 98.8 °C, 0.724 centistokes. |
| Odor Threshold | Odor Threshold Low: 1.1 [mmHg] Odor threshold from CHEMINFO |
| Refractive Index | Index of refraction: 1.5542 @ 20 °C/D |
| Kovats Retention Index | 1196.1 1236.7 1208 1223 1224.7 1199.1 1213.2 1222.1 1206 1222 |
| Other Experimental Properties | Compatible with numerous resins. |
| Chemical Classes | Other Classes -> Halogenated Aliphatics, Unsaturated |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H310:Acute toxicity,dermal H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H351:Carcinogenicity H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 260.8 g/mol |
|---|---|
| XLogP3 | 4.8 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 0 |
| Rotatable Bond Count | 1 |
| Exact Mass | 259.810166 g/mol |
| Monoisotopic Mass | 257.813116 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 160 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
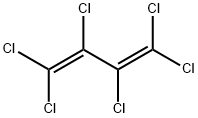
Hexachlorobutadiene is a colorless liquid with a turpentine-like odor. It is also called perchlorobutadiene. Hexachlorobutadiene is not found naturally in the environment. It is formed when other chemicals are made. Most hexachlorobutadiene used commercially in the United States is imported from Germany. It is mainly used to make rubber compounds. It is also used as a solvent, and to make lubricants, in gyroscopes, as a heat transfer liquid, and as a hydraulic fluid.