78183-34-3
Product Name:
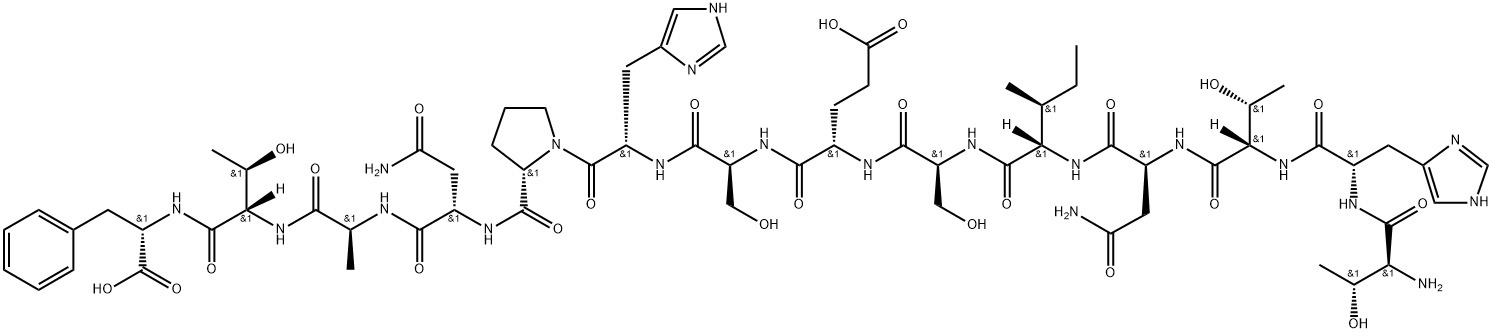
L-Threonyl-L-histidyl-L-threonyl-L-asparaginyl-L-isoleucyl-L-seryl-L-alpha-glutamyl-L-seryl-L-histidyl-L-prolyl-L-asparaginyl-L-alanyl-L-threonyl-L-phenylalanine
Formula:
C66H98N20O24
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Colorless solid; [Merck Index] |
|---|---|
| Chemical Classes | Metals -> Metalloid Compounds (Boron) |
COMPUTED DESCRIPTORS
| Molecular Weight | 253.9 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 1 |
| Exact Mass | 254.1860696 g/mol |
| Monoisotopic Mass | 254.1860696 g/mol |
| Topological Polar Surface Area | 36.9 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 286 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Bis(pinacolato)diboron is a 1,3,2-dioxaborolane that is 4,4,5,5-tetramethyl-1,3,2-dioxaborolane in which the boron hydrogen at position 2 is replaced by a 4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl group. It is a reagent used in organic synthesis to synthesise pinacol boronic esters. It has a role as an EC 3.4.24.24 (gelatinase A) inhibitor.