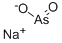CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Sodium arsenite, aqueous solution appears as an aqueous solution of a solid. Toxic by ingestion, inhalation or skin absorption. Used as an antiseptic, in insecticides and herbicides, to preserve hides and in making dyes. |
|---|---|
| Color/Form | White or grayish-white powder |
| Taste | SALTY TASTE |
| Melting Point | 615 °C |
| Solubility | Very soluble (NTP, 1992) |
| Density | 1.87 at 68 °F (NTP, 1992) - Denser than water; will sink |
| Stability/Shelf Life | SLOWLY CONVERTED IN SOLN TO ARSENATES BY ATMOSPHERIC OXYGEN; IN DRY STATE DECOMP BY ATMOSPHERIC CO2. /ARSENITES OF ALKALI METALS/ |
| Decomposition | When heated to decomposition it emits toxic fumes of /arsenic and disodium oxide/. |
| Other Experimental Properties | Somewhat hygroscopic; absorbs CO2 from air |
| Chemical Classes | Metals -> Arsenic Compounds, Inorganic |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H350:Carcinogenicity H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
COMPUTED DESCRIPTORS
| Molecular Weight | 129.910 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 129.901193 g/mol |
| Monoisotopic Mass | 129.901193 g/mol |
| Topological Polar Surface Area | 40.1 Ų |
| Heavy Atom Count | 4 |
| Formal Charge | 0 |
| Complexity | 13.5 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Sodium arsenite, aqueous solution appears as an aqueous solution of a solid. Toxic by ingestion, inhalation or skin absorption. Used as an antiseptic, in insecticides and herbicides, to preserve hides and in making dyes.