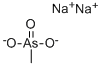CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Colorless hygroscopic solid; [Hawley] |
|---|---|
| Color/Form | Hydrated crystals containing 5H2O or 6H2O |
| Taste | Pleasant acid taste |
| Melting Point | >355 °C |
| Solubility | Soluble in methanol; practically insoluble in most organic solvents |
| Density | 1.04 g/ml |
| Vapor Pressure | 0.0000001 [mmHg] |
| LogP | Kow = <10 |
| Stability/Shelf Life | DECOMPOSED BY STRONG OXIDIZING & REDUCING AGENTS. |
| Decomposition | When heated to decomposition it emits toxic fumes of /arsenic & sodium oxide/. |
| Corrosivity | NON-CORROSIVE TO IRON, RUBBER AND MOST PLASTICS |
| Dissociation Constants | pKa1 = 4.1; pKa2 = 8.94 |
| Other Experimental Properties | Hydrolysis to MSMA @ pH 6-7; decomposed by strong oxidizing and reducing agents |
| Chemical Classes | Metals -> Arsenic Compounds, Organic |
COMPUTED DESCRIPTORS
| Molecular Weight | 183.934 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 183.909352 g/mol |
| Monoisotopic Mass | 183.909352 g/mol |
| Topological Polar Surface Area | 63.2 Ų |
| Heavy Atom Count | 7 |
| Formal Charge | 0 |
| Complexity | 49.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 3 |
| Compound Is Canonicalized | Yes |