7681-52-9
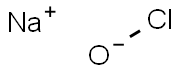
Product Name:
Sodium hypochlorite
Formula:
ClNaO
Synonyms:
bleach;Soda bleaching lye, Eau de Labarraque
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Sodium hypochlorite appears as colorless or slightly yellow watery liquid with an odor of household bleach. Mixes with water. (USCG, 1999) |
|---|---|
| Color/Form | Greenish yellow liquid |
| Odor | Disagreeable, sweetish odor |
| Boiling Point | Boiling point: 111 °C in solution |
| Melting Point | MP: 18 °C /Pentahydrate/ |
| Solubility | 29.3 g/100 g (0 °C) in water |
| Density | 1.093 for 5% solution (USCG, 1999) - Denser than water; will sink |
| Vapor Pressure | Vapor pressure, kPa at 20 °C: 2 - 2.5 |
| Stability/Shelf Life | UNSTABLE IN AIR UNLESS MIXED WITH SODIUM HYDROXIDE. |
| Decomposition | The anhydrous solid obtained by dessication of the /sodium hypochlorite/ pentahydrate will decomp violently on heating or friction. |
| Odor Threshold | The estimated odor threshold is the same as chlorine, 0.3 ppm |
| Other Experimental Properties | Does not produce red color with phenolphthalein; colorless or slightly yellow liq /Sodium hypochlorite soln, diluted/ |
| Chemical Classes | Other Uses -> Biocides/Disinfectants |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Corrosion Corrosives GHS05  Environment GHS09 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P363:Wash contaminated clothing before reuse. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 74.44 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 73.9535366 g/mol |
| Monoisotopic Mass | 73.9535366 g/mol |
| Topological Polar Surface Area | 23.1 Ų |
| Heavy Atom Count | 3 |
| Formal Charge | 0 |
| Complexity | 4.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Sodium hypochlorite, the active ingredient in chlorine bleach, is routinely used in the laboratory to decontaminate surfaces and equipment or deactivate biological materials by inactivating vegetative bacteria, fungi, lipid and non-lipid viruses, and other liquid specimens.
RELATED SUPPLIERS
All suppliers(51)
