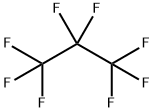
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Octafluoropropane is a colorless, odorless gas. It is relatively inert. The mixture is nonflammable and nontoxic, though asphyxiation may occur because of displacement of oxygen. Exposure of the container to prolonged heat or fire can cause it to rupture violently and rocket. |
|---|---|
| Color/Form | Colorless, nonflammable gas |
| Boiling Point | -36.6 °C |
| Melting Point | -147.6 °C |
| Solubility | 5.7 mg/L (at 15 °C) |
| Density | 1.352 at 20 °C (liquid) |
| Vapor Pressure | 6.63X10+3 mm Hg at 25 °C |
| LogP | 3 |
| Other Experimental Properties | Ozone Depletion Potential = 0 |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Gas Cylinder Compressed Gases GHS04  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H280:Gases under pressure H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
COMPUTED DESCRIPTORS
| Molecular Weight | 188.02 g/mol |
|---|---|
| XLogP3 | 3.1 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 0 |
| Exact Mass | 187.98722530 g/mol |
| Monoisotopic Mass | 187.98722530 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 120 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Octafluoropropane is a colorless, odorless gas. It is relatively inert. The mixture is nonflammable and nontoxic, though asphyxiation may occur because of displacement of oxygen. Exposure of the container to prolonged heat or fire can cause it to rupture violently and rocket.