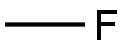CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Methyl fluoride (or fluoromethane) is a colorless flammable gas which is heavier than air. It has an agreeable ether-like odor. It is narcotic in high concentrations. It burns with evolution of hydrogen fluoride. The flame is colorless, similar to alcohol. Under prolonged exposure to fire or intense heat the containers may rupture violently and rocket. |
|---|---|
| Kovats Retention Index | 217 |
| Chemical Classes | Solvents -> Chlorofluorocarbons |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Flame Flammables GHS02  Gas Cylinder Compressed Gases GHS04 |
| GHS Hazard Statements |
H220:Flammable gases H280:Gases under pressure H336:Specific target organ toxicity,single exposure; Narcotic effects |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
COMPUTED DESCRIPTORS
| Molecular Weight | 34.033 g/mol |
|---|---|
| XLogP3 | 0.6 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 34.021878258 g/mol |
| Monoisotopic Mass | 34.021878258 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 2 |
| Formal Charge | 0 |
| Complexity | 2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Methyl fluoride (or fluoromethane) is a colorless flammable gas which is heavier than air. It has an agreeable ether-like odor. It is narcotic in high concentrations. It burns with evolution of hydrogen fluoride. The flame is colorless, similar to alcohol. Under prolonged exposure to fire or intense heat the containers may rupture violently and rocket.