CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | White solid; Sensitive to light; [Merck Index] Colorless solid; [MSDSonline] |
|---|---|
| Color/Form | WHITE POWDER |
| Melting Point | 105-105.6 °C |
| Solubility | INSOL IN WATER; 1.5% IN METHANOL; 7% IN XYLENE |
| Vapor Pressure | 0.00000011 [mmHg] |
| Stability/Shelf Life | LIGHT-SENSITIVE BUT DISCOLORATION DOES NOT AFFECT ITS BIOLOGICAL ACTIVITY |
| Decomposition | Decomposed by alkaline media; DT50 (22 °C) >15 days (pH = 4), >18 hours (pH = 7), < 10 minutes (pH = 9), and by polysulfides. Light sensitive. |
| Ionization Efficiency | Positive |
| Kovats Retention Index | 1921 1943 1930.8 1929.6 1916.5 1946.5 |
| Chemical Classes | Pesticides -> Fungicides |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H332:Acute toxicity,inhalation H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 333.2 g/mol |
|---|---|
| XLogP3 | 3.6 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 5 |
| Exact Mass | 331.9623035 g/mol |
| Monoisotopic Mass | 331.9623035 g/mol |
| Topological Polar Surface Area | 74.3 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 367 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
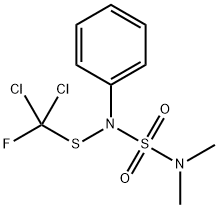
Dichlofluanid is a member of the class of sulfamides that is sulfamide in which the hydrogens attached to one of the nitrogens are replaced by methyl groups, while those attached to the other nitrogen are replaced by a phenyl and a [dichloro(fluoro)methyl]sulfanediyl group. A fungicide introduced in 1965 and used in the cultivation of fruit and vegetables, as well as in wood preservatives, it is no longer approved for use in the European Union. It has a role as an acaricide and an antifungal agrochemical. It is a member of sulfamides, an organofluorine compound, an organochlorine compound and a phenylsulfamide fungicide. It is functionally related to a sulfamide.