COMPUTED DESCRIPTORS
| Molecular Weight | 305.32 g/mol |
|---|---|
| XLogP3 | 1.4 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 1 |
| Exact Mass | 305.12632271 g/mol |
| Monoisotopic Mass | 305.12632271 g/mol |
| Topological Polar Surface Area | 90.2 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 479 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
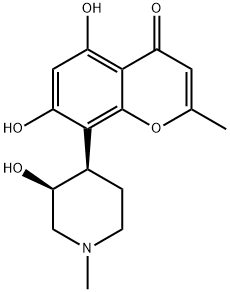
Rohitukine is a member of the class of chromones that is 4H-chromen-4-one in which the hydrogens at positions 2,5,7 and 8 are replaced by methyl, hydroxy, hydroxy, and (3S,4R)-3-hydroxy-1-methylpiperidin-4-yl groups, respectively. It is an alkaloid initially isolated from Amoora rohituka and is a precursor of the anti-cancer compound flavopiridol. It has a role as a plant metabolite, an antineoplastic agent, an EC 2.7.11.22 (cyclin-dependent kinase) inhibitor, a fungal metabolite, an anti-inflammatory agent, an anti-ulcer drug, an anticholesteremic drug and an antileishmanial agent. It is a hydroxypiperidine, a tertiary amino compound, a member of chromones, a member of resorcinols and an alkaloid.