131740-09-5
Product Name:
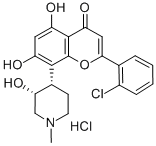
FLAVOPIRIDOL HYDROCHLORIDE
Formula:
C21H21Cl2NO5
Synonyms:
(−)-2-(2-Chlorophenyl)-5,7-dihydroxy-8-[(3S,4R)-3-hydroxy-1-methyl-4-piperidinyl]-4H-1-benzopyran-4-one hydrochloride;L-86-8276;NSC-649890
Inquiry
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 438.3 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 2 |
| Exact Mass | 437.0796782 g/mol |
| Monoisotopic Mass | 437.0796782 g/mol |
| Topological Polar Surface Area | 90.2 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 628 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Alvocidib hydrochloride is a hydrochloride salt resulting from the formal reaction of equimolar amounts of alvocidib and hydrogen chloride. A cyclin-dependent kinase 9 (CDK9) inhibitor, it has been studied for the treatment of acute myeloid leukaemia, arthritis and atherosclerotic plaque formation. It has a role as an antineoplastic agent, an antirheumatic drug, an apoptosis inducer and an EC 2.7.11.22 (cyclin-dependent kinase) inhibitor. It contains an alvocidib(1+).
