62013-04-1
Product Name:
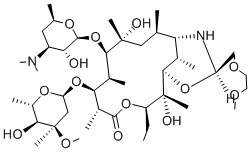
Dirithromycin
Formula:
C42H78N2O14
Synonyms:
[9S(R)]-9-Deoxo-11-deoxy-9,11-[imino[2-(2-methoxy)ethylidene]oxy]erthromycin; LY-237216
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Solubility | Poor |
|---|---|
| LogP | 1.6 |
| Collision Cross Section | 284.2 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards] |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H317:Sensitisation, Skin |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. |
COMPUTED DESCRIPTORS
| Molecular Weight | 835.1 g/mol |
|---|---|
| XLogP3 | 4.2 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 16 |
| Rotatable Bond Count | 12 |
| Exact Mass | 834.54530517 g/mol |
| Monoisotopic Mass | 834.54530517 g/mol |
| Topological Polar Surface Area | 196 Ų |
| Heavy Atom Count | 58 |
| Formal Charge | 0 |
| Complexity | 1300 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 20 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Dirithromycin is the hemi-aminal resulting from the condensation of the erythromycin derivative (9S)-erythromycyclamine with 2-(2-methoxyethoxy)acetaldehyde. As the oxazine ring containing the hemi-aminal group is unstable under both acidic and alkaline conditions, dirithromycin functions as a more lipid-soluble prodrug for (9S)-erythromycyclamine. Administered as enteric coated tablets to protect it from acid catalysed hydrolysis in the stomach, it is used to treat respiratory tract, skin, and soft tissue infections caused by susceptible organisms. It has a role as a prodrug.
