CHEMICAL AND PHYSICAL PROPERTIES
| Collision Cross Section | 180 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards] |
|---|
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P262:Do not get in eyes, on skin, or on clothing. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
COMPUTED DESCRIPTORS
| Molecular Weight | 367.4 g/mol |
|---|---|
| XLogP3 | 2.6 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 1 |
| Exact Mass | 367.10558726 g/mol |
| Monoisotopic Mass | 367.10558726 g/mol |
| Topological Polar Surface Area | 66.5 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 615 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
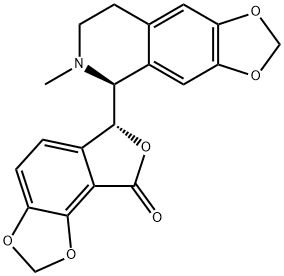
Bicuculline is a benzylisoquinoline alkaloid that is 6-methyl-5,6,7,8-tetrahydro[1,3]dioxolo[4,5-g]isoquinoline which is substituted at the 5-pro-S position by a (6R)-8-oxo-6,8-dihydrofuro[3,4-e][1,3]benzodioxol-6-yl group. A light-sensitive competitive antagonist of GABAA receptors. It was originally identified in 1932 in plant alkaloid extracts and has been isolated from Dicentra cucullaria, Adlumia fungosa, Fumariaceae, and several Corydalis species. It has a role as an agrochemical, a central nervous system stimulant, a GABA-gated chloride channel antagonist, a neurotoxin and a GABAA receptor antagonist. It is an isoquinoline alkaloid, a member of isoquinolines and a benzylisoquinoline alkaloid.