32795-47-4
Product Name:
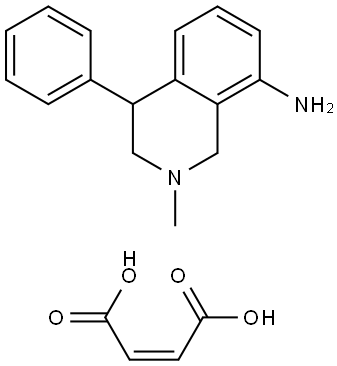
NOMIFENSINE MALEATE SALT
Formula:
C16H18N2.C4H4O4
Synonyms:
1,2,3,4-Tetrahydro-2-methyl-4-phenyl-8-isoquinolinamine maleate salt
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Collision Cross Section | 155.5 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards] |
|---|
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 354.4 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 3 |
| Exact Mass | 354.15795719 g/mol |
| Monoisotopic Mass | 354.15795719 g/mol |
| Topological Polar Surface Area | 104 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 391 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
An isoquinoline derivative that prevents dopamine reuptake into synaptosomes. The maleate was formerly used in the treatment of depression. It was withdrawn worldwide in 1986 due to the risk of acute hemolytic anemia with intravascular hemolysis resulting from its use. In some cases, renal failure also developed. (From Martindale, The Extra Pharmacopoeia, 30th ed, p266)