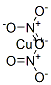CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Obtained as a trihydrate and as a hexahydrate. Both are blue crystalline solids. Used in medicine, as an insecticide, in chemical analysis, in making light sensitive papers. Toxic oxides of nitrogen are produced in fires involving this material. |
|---|---|
| Color/Form | Large, blue-green, orthorhombic crystals |
| Boiling Point | Sublimes |
| Melting Point | 238.1 °F (USCG, 1999) |
| Solubility | 137.8 g/100 cc water @ 0 °C, 1270 g/100 cc water @ 100 °C, 100 g/100 cc alcohol @ 12.5 °C, very slightly sol in liq ammonia /Cupric nitrate trihydrate/ |
| Density | 2.32 at 68 °F (USCG, 1999) - Denser than water; will sink |
| Other Experimental Properties | Sublimes @ 150-225 °C |
| Chemical Classes | Metals -> Metals, Inorganic Compounds |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids H315:Skin corrosion/irritation H318:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P220:Keep/Store away from clothing/…/combustible materials. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P501:Dispose of contents/container to..… |
COMPUTED DESCRIPTORS
| Molecular Weight | 187.56 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 0 |
| Exact Mass | 186.905233 g/mol |
| Monoisotopic Mass | 186.905233 g/mol |
| Topological Polar Surface Area | 126 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 18.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 3 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Obtained as a trihydrate and as a hexahydrate. Both are blue crystalline solids. Used in medicine, as an insecticide, in chemical analysis, in making light sensitive papers. Toxic oxides of nitrogen are produced in fires involving this material.