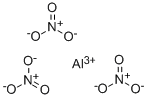
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Aluminum nitrate appears as a white, crystalline solid. Noncombustible but can accelerate the burning of combustible materials. If large quantities are involved or the combustible material is finely divided, an explosion may result. Prolonged exposure to fire or heat may result in an explosion. Fires that involve this material, produce oxides of nitrogen. Uses include, petroleum refining, dyeing, and leather tanning. |
|---|---|
| Melting Point | 163 °F (USCG, 1999) |
| Density | greater than 1 at 68 °F (USCG, 1999) |
| Stability/Shelf Life | Nonahydrate /state/ is the most stable. /Nonahydrate/ |
| Decomposition | On decomposition it emits toxic /nitrogen oxides/. |
| Odor Threshold | Odor Threshold Low: 0.01 [mg/m3] [CAMEO] Odor threshold from HSDB |
| Refractive Index | Colorless, rhombic crystals. Index of refraction: 1.54. /Nonahydrate/ |
| Other Experimental Properties | Deliquescent crystals; mp 73 °C; decomposes @ 135 °C. Very soluble in water, alcohol; very slightly soluble in acetone. Almost insoluble in ethyl acetate and pyridine. The aqueous solution is acid. /Nonahydrate/ |
| Chemical Classes | Metals -> Metals, Inorganic Compounds |
COMPUTED DESCRIPTORS
| Molecular Weight | 213.00 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 0 |
| Exact Mass | 212.9449920 g/mol |
| Monoisotopic Mass | 212.9449920 g/mol |
| Topological Polar Surface Area | 189 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 18.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 4 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Aluminum nitrate appears as a white, crystalline solid. Noncombustible but can accelerate the burning of combustible materials. If large quantities are involved or the combustible material is finely divided, an explosion may result. Prolonged exposure to fire or heat may result in an explosion. Fires that involve this material, produce oxides of nitrogen. Uses include, petroleum refining, dyeing, and leather tanning.