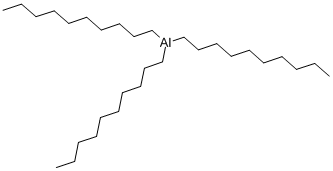
CHEMICAL AND PHYSICAL PROPERTIES
| Stability/Shelf Life | Low melting solids or colorless, volatile liquids. /Alkylaluminum halides/ |
|---|---|
| Other Experimental Properties | Normal (linear) alkylaluminum compounds exist largely as associated dimers at 25 °C, with bridging alkyl groups. |
| Chemical Classes | Metals -> Metals, Organic Compounds |
COMPUTED DESCRIPTORS
| Molecular Weight | 450.8 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 0 |
| Rotatable Bond Count | 27 |
| Exact Mass | 450.4745154 g/mol |
| Monoisotopic Mass | 450.4745154 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 253 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Tri-n-decylaluminum is an organoaluminum compound. Aluminum is the most abundant metal in the earth's crust and is always found combined with other elements such as oxygen, silicon, and fluorine. (L739, L740)