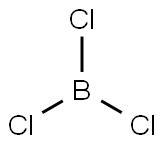
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Boron trichloride appears as a colorless gas with a pungent odor. Fumes irritate the eyes and mucous membranes. Corrosive to metals and tissue and is toxic. Under prolonged exposure to fire or intense heat, the containers may rupture violently and rocket. Used as a catalyst in chemical manufacture, in soldering fluxes, and for many other uses. |
|---|---|
| Color/Form | Gas at room temperature |
| Odor | Pungent, irritating odor |
| Boiling Point | 54.5 °F at 760 mmHg (EPA, 1998) |
| Melting Point | -161 °F (EPA, 1998) |
| Solubility | Solubility in water: reaction |
| Density | 1.35 at 53.6 °F (EPA, 1998) - Denser than water; will sink |
| Vapor Density | 4.03 (EPA, 1998) - Heavier than air; will sink (Relative to Air) |
| Vapor Pressure | 760 mmHg at 54.86 °F (EPA, 1998) |
| Stability/Shelf Life | Trialkylboranes are stable indefinitely when stored under an inert atmosphere. /Trialkylboranes/ |
| Decomposition | When heated to decomposition it emits toxic fumes of /hydrogens of chloride/. |
| Viscosity | 1.032 cP at 50 °F |
| Corrosivity | It is corrosive to metals |
| Heat of Vaporization | 23.77 kJ/mol at 12.65 °C; 23.1 kJ/mol at 25 °C |
| Surface Tension | 16.7 dynes/cm = 0.0167 N/m at 20 °C |
| Refractive Index | INDEX OF REFRACTION: 1.4195 AT 5.7 °C ALPHA LINE H2 |
| Other Experimental Properties | Colorless fuming liquid at low temperature |
| Chemical Classes | Toxic Gases & Vapors -> Corrosive Gases |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H300:Acute toxicity,oral H314:Skin corrosion/irritation H370:Specific target organ toxicity, single exposure |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 117.2 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 0 |
| Rotatable Bond Count | 0 |
| Exact Mass | 115.915863 g/mol |
| Monoisotopic Mass | 115.915863 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 4 |
| Formal Charge | 0 |
| Complexity | 8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Boron trichloride appears as a colorless gas with a pungent odor. Fumes irritate the eyes and mucous membranes. Corrosive to metals and tissue and is toxic. Under prolonged exposure to fire or intense heat, the containers may rupture violently and rocket. Used as a catalyst in chemical manufacture, in soldering fluxes, and for many other uses.
RELATED SUPPLIERS
Sainor Laboratories Pvt Ltd Unit III
1Y
product:Boron trichloride 1.0M in MDC/ 1.0M in n-Hexane / 1.0M in P-xylene 1.0M