7720-78-7
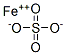
Product Name:
Ferrous sulfate
Formula:
FeO4S
Synonyms:
1,10-Phenanthroline iron(II) salt solution;Ferrous sulfate heptahydrate;Iron(II) sulfate heptahydrate
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Ferrous sulfate appears as a greenish or yellow-brown crystalline solid. Density 15.0 lb /gal. Melts at 64 °C and loses the seven waters of hydration at 90 °C. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Used for water or sewage treatment, as a fertilizer ingredient. |
|---|---|
| Color/Form | White orthorhombic crystals, hygroscopic |
| Boiling Point | > 300 °C |
| Melting Point | 64 |
| Solubility | 25.6 g/100 mL |
| Density | 1.9 at 59 °F (USCG, 1999) - Denser than water; will sink |
| LogP | -0.84 |
| Stability/Shelf Life | In moist air, ferrous sulfate rapidly oxidizes and becomes coated with brownish-yellow ferric sulfate... . The rate of oxidation is increased by the addition of alkali or by exposure to light. |
| Autoignition Temperature | Not flammable (USCG, 1999) |
| Decomposition | When heated to decomposition it emit toxic fumes of /sulfur oxide/. |
| Refractive Index | INDEX OF REFRACTION: 1.471, 1.478, 1.486; MP 64 °C LOSING 6H2O; BP 300 °C LOSING 7H2O. /FERROUS SULFATE HEPTAHYDRATE/ |
| Dissociation Constants | -3 |
| Other Experimental Properties | Standard molar enthalpy (heat) of formation at 298.15 deg K is -928.4 kJ/mol (crystal); Standard molar Gibbs energy of formation at 298.15 deg K is -820.8 kJ/mol (crystal); Standard molar entropy at 298.15 deg K is 107.5 J/mol K (crystal); Molar heat capacity at constant pressure at 298.15 deg K is 100.6 J/mol K (crystal) |
| Chemical Classes | Metals -> Metals, Inorganic Compounds |
COMPUTED DESCRIPTORS
| Molecular Weight | 151.91 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 151.886665 g/mol |
| Monoisotopic Mass | 151.886665 g/mol |
| Topological Polar Surface Area | 88.6 Ų |
| Heavy Atom Count | 6 |
| Formal Charge | 0 |
| Complexity | 62.2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Ferrous sulfate appears as a greenish or yellow-brown crystalline solid. Density 15.0 lb /gal. Melts at 64 °C and loses the seven waters of hydration at 90 °C. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Used for water or sewage treatment, as a fertilizer ingredient.
RELATED SUPPLIERS
Canton Laboratories Private Limited
Vadodara
product:Powder Dried Ferrous Sulphate, Grade Standard: Bio-Tech Grade
