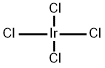
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Brownish-black solid; Soluble in water; [Hawley] |
|---|---|
| Color/Form | Brownish-black mass |
| Solubility | Soluble in water, alcohol, and dilute hydrochloric acid. |
| Decomposition | When heated to decomposition it emits toxic fumes of /hydrogen chloride/. |
| Other Experimental Properties | /The chloride of iridium(IV) ion most likely does not exist as IrCl4./ Iridium(IV) ion tends to be six-cooridinate with octahedral geometry. |
| Chemical Classes | Metals -> Metals, Inorganic Compounds |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P330:Rinse mouth. |
COMPUTED DESCRIPTORS
| Molecular Weight | 334.0 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 0 |
| Rotatable Bond Count | 0 |
| Exact Mass | 334.83538 g/mol |
| Monoisotopic Mass | 332.83833 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 19.1 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |