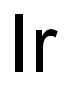CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Liquid, Other Solid |
|---|---|
| Color/Form | Silver-white, face-centered cubic lattice |
| Boiling Point | 4,428 °C |
| Melting Point | 2,446 °C |
| Density | 22.42 at 17 °C |
| Vapor Pressure | 0.467 Pa at melting point |
| Corrosivity | Not attacked by any acid or by aqua regia. |
| Other Experimental Properties | Powdered iridium metal can ignite in air. |
| Chemical Classes | Metals -> Elements, Metallic |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Flame Flammables GHS02 |
| GHS Hazard Statements |
H228:Flammable solids |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P370+P378:In case of fire: Use … for extinction. |
COMPUTED DESCRIPTORS
| Molecular Weight | 192.22 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 0 |
| Rotatable Bond Count | 0 |
| Exact Mass | 192.96292 g/mol |
| Monoisotopic Mass | 192.96292 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 1 |
| Formal Charge | 0 |
| Complexity | 0 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Iridium atom is a cobalt group element atom and a platinum group metal atom.