10421-48-4
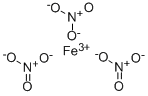
Product Name:
Ferric nitrate
Formula:
FeN3O9
Synonyms:
Iron(III) nitrate solution
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Ferric nitrate appears as a violet crystalline solid. Noncombustible but it will accelerate the burning of combustible materials. If large quantities are involved in the fire or the combustible material is finely divided an explosion may result. Prolonged exposure of the material to fire or heat may result in an explosion. Toxic oxides of nitrogen are produced in fires involving this material. It is used for dyeing and tanning, in chemical analysis, and in medicine. |
|---|---|
| Melting Point | 117 °F (USCG, 1999) |
| Solubility | Solubility in cold water: 150 g/100 cc; soluble in all proportions in hot water. /Ferric nitrate hexahydrate/ |
| Density | 1.7 at 68 °F (USCG, 1999) - Denser than water; will sink |
| Other Experimental Properties | Solutions are corrosive to most metals. /Ferric nitrate nonahydrate/ |
| Chemical Classes | Metals -> Metals, Inorganic Compounds |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H290:Corrosive to Metals H315:Skin corrosion/irritation H318:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P234:Keep only in original container. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. |
COMPUTED DESCRIPTORS
| Molecular Weight | 241.86 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 0 |
| Exact Mass | 241.898389 g/mol |
| Monoisotopic Mass | 241.898389 g/mol |
| Topological Polar Surface Area | 189 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 18.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 4 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Ferric nitrate appears as a violet crystalline solid. Noncombustible but it will accelerate the burning of combustible materials. If large quantities are involved in the fire or the combustible material is finely divided an explosion may result. Prolonged exposure of the material to fire or heat may result in an explosion. Toxic oxides of nitrogen are produced in fires involving this material. It is used for dyeing and tanning, in chemical analysis, and in medicine.