10377-60-3
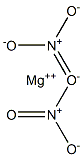
Product Name:
Magnesium nitrate
Formula:
MgN2O6
Synonyms:
Magnesium modifier;Magnesium nitrate – nitric acid solution
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Magnesium nitrate appears as a white crystalline solid. Produces toxic oxides of nitrogen if heated to decomposition. Used in pyrotechnics. |
|---|---|
| Color/Form | White cubic crystals |
| Boiling Point | Decomposes at 626 °F (USCG, 1999) |
| Melting Point | 192 °F (USCG, 1999) |
| Solubility | Very soluble in water |
| Density | 1.46 (USCG, 1999) - Denser than water; will sink |
| Vapor Pressure | 0.49 [mmHg] |
| Decomposition | WHEN HEATED TO DECOMP ... EMITS TOXIC FUMES OF /NITROGEN OXIDES/. |
| Other Experimental Properties | Soluble in liquid ammonia /Hexahydrate/ |
| Chemical Classes | Metals -> Metals, Inorganic Compounds |
SAFETY INFORMATION
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P220:Keep/Store away from clothing/…/combustible materials. P221:Take any precaution to avoid mixing with combustibles/… P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
COMPUTED DESCRIPTORS
| Molecular Weight | 148.32 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 0 |
| Exact Mass | 147.9606774 g/mol |
| Monoisotopic Mass | 147.9606774 g/mol |
| Topological Polar Surface Area | 126 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 18.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 3 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Magnesium nitrate appears as a white crystalline solid. Produces toxic oxides of nitrogen if heated to decomposition. Used in pyrotechnics.