10048-95-0
Product Name:
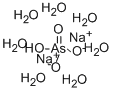
SODIUM ARSENATE, HEPTAHYDRATE
Formula:
AsH15Na2O11
Synonyms:
Disodium hydrogen arsenate heptahydrate;di-Sodium hydrogen arsenate heptahydrate
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Sodium arsenate heptahydrate appears as odorless white crystals. Becomes anhydrous at 212 °F. Aqueous solution is alkaline to litmus. (NTP, 1992) |
|---|---|
| Boiling Point | 356 °F at 760 mmHg (decomposes) (NTP, 1992) |
| Melting Point | 135 °F (NTP, 1992) |
| Solubility | greater than or equal to 100 mg/mL at 77 °F (NTP, 1992) |
| Density | 1.88 (NTP, 1992) - Denser than water; will sink |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H350:Carcinogenicity H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
COMPUTED DESCRIPTORS
| Molecular Weight | 312.01 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 8 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 0 |
| Exact Mass | 311.962569 g/mol |
| Monoisotopic Mass | 311.962569 g/mol |
| Topological Polar Surface Area | 90.4 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 46.5 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 10 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Sodium arsenate heptahydrate appears as odorless white crystals. Becomes anhydrous at 212 °F. Aqueous solution is alkaline to litmus. (NTP, 1992)