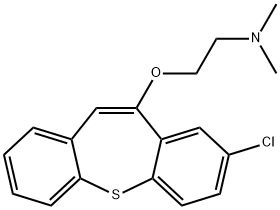
ZOTEPINE
Synonym(s):2-[(8-chlorodibenzo[b,f]-thiepin-10-yl)oxy]-N,N-dimethylethanamine
- CAS NO.:26615-21-4
- Empirical Formula: C18H18ClNOS
- Molecular Weight: 331.86
- MDL number: MFCD00868143
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
What is ZOTEPINE?
Absorption
Preclinical pharmacokinetic studies have shown a dose-dependent increase in plasma levels with a tmax between 2-4 hours and Cmax from 6.9-19.6 ng/ml when administered in a dose of 25-100 mg of zotepine. The maximum concentration peaks and slow declines thereafter. When administered orally in preclinical studies, zotepine was proven to be absorbed rapidly and almost completely from the gastrointestinal tract.The unchanged drug and metabolites are rapidly distributed to the tissues.
Toxicity
The use of zotepine has been vastly related to ongoing extrapyramidal side effects and it has been reported to be poorly tolerated by the patients.
Chemical properties
White to off white crystalline powder
Originator
Lodopin,Fujisawa Pharmaceutical,Japan,1982
The Uses of ZOTEPINE
antipsychotic;Dopamine D2/serotonin 5-HT2 antagonist
Indications
Zotepine, like other atypical antipsychotics, is considered as the first-line treatment in newly diagnosed schizophrenia. It is usually thought to be an option of choice for managing acute schizophrenic episodes when discussion with the patient is not possible. Zotepine, as an atypical antipsychotic, is used in patients who are suffering unacceptable side effects from conventional antipsychotics or in relapse patients that were inadequately controlled.
It is important to consider that the indications stated above are related to atypical antipsychotics, that zotepine is not currently FDA, Canada or EMA approved and that studies have not shown any additional benefit when compared with other approved atypical antipsychotics.
Schizophrenia is a chronic and severe mental disorder that affects how a person thinks, feels and behaves. It is usually marked for a loose reality perspective delineated by hallucinations, delusions and thought and movement disorders.
Background
Zotepine, with the formula (2-chloro-11-(2-dimethyl-amino-ethoxy)-dibenzo thiepin, is a neuroleptic drug. It was designed and synthesized by Fujisawa Pharmaceutical Co Ltd. It has been used as an antipsychotic in Japan, India and some places in Europe like UK and Germany since 1980's. Zotepine was never approved by the FDA. In 1993, it was classified as inactive drug substance (Status I, Type II) and in 1995 the FDA studied the manufacturing procedures of Zotepine tablets in Germany, but the status remained inactive. When the analysis of antipsychotics was retaken in 2016 by the FDA, zotepine did not reach the threshold effect to be further studied.. In the EMA, by 2015 it was under pharmacovigilance studies for the potential treatment of acute renal failure.
What are the applications of Application
Zotepine is an SR-2A and D2DR inhibitor and Histamine H1 receptor antagonist
Definition
ChEBI: Zotepine is a dibenzothiepine and a tertiary amino compound. It has a role as an alpha-adrenergic drug, a serotonergic drug and a second generation antipsychotic.
Manufacturing Process
A suspension of 30 g of sodium hydride in benzene (30 ml) was added dropwise to 52 g of 8-chlorodibenzo[b,f]thiepin-10(11H)-one dissolved in dimethylformamide (800 ml), and the mixture was heated at 100°C for 2 hours. To this, there were added 68 g of 2-dimethylaminoethyl chloride, and the mixture was heated at 60°C for 39 hours. The reaction mixture, after cooled, was poured into ice-water, and the solution was extracted with ethyl acetate. The ethyl acetate layer, after washed with water, was extracted with 10% hydrochloric acid, when oil was precipitated. The aqueous layer, in which oil was precipitated, was washed with ether, made neutral with concentrated sodium hydroxide solution and then extracted with ethyl acetate. The ethyl acetate layer was washed with water, dried over magnesium sulfate, and concentrated to give oil, which was allowed to stand to provide solid. The solid was washed with petroleum ether and recrystallized from cyclohexane to yield 42.5 g of 8-chloro-10-(2-dimethylaminoethyl)-oxydibenzo[b,f]thiepin as crystals, melting point 90°C to 91°C. Maleate as colorless needle, melting point 204°C to 204.5°C.
Therapeutic Function
Tranquilizer
Pharmacokinetics
In preclinical studies, zotepine is characterized by the presence of a strong antiserotonergic activity when compared with other neuroleptic drugs. It has also been reported to present elevations in the seizure threshold in the amygdaloid nucleus. When zotepine's effects were analyzed by electroencephalography, it was noted a typical response of a low-potency neuroleptics of the sedative type. Administration of zotepine has shown improvements in numerical movements and complex reaction. These effects tend to be accompanied by increases in pulse rate, increase in prolactin levels and some typical neuroleptic side effects.
Metabolism
Zotepine is well metabolized in the body, it actually undergoes extensive first-pass metabolism to the metabolite norzotepine and several inactive metabolites. The main enzymes involved in zotepine metabolism are CYP1A2 and CYP3A4. Some of the main metabolic pathways include N-demethylation and oxygenation of N or S atoms, hydroxylation of the aromatic ring and consecutive conjugation.
Properties of ZOTEPINE
| Melting point: | 90-91° |
| Boiling point: | 478.4±45.0 °C(Predicted) |
| Density | 1.0675 (rough estimate) |
| refractive index | 1.6100 (estimate) |
| storage temp. | Store at RT |
| solubility | DMSO: ≥20mg/mL |
| form | powder |
| pka | 8.72±0.28(Predicted) |
| color | white to off-white |
Safety information for ZOTEPINE
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P391:Collect spillage. Hazardous to the aquatic environment P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P405:Store locked up. |
Computed Descriptors for ZOTEPINE
ZOTEPINE manufacturer
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 26615-21-4 Zotepine 98%View Details
26615-21-4 Zotepine 98%View Details
26615-21-4 -
 Zotepine 95.00% CAS 26615-21-4View Details
Zotepine 95.00% CAS 26615-21-4View Details
26615-21-4 -
 Zotepine CAS 26615-21-4View Details
Zotepine CAS 26615-21-4View Details
26615-21-4 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8
